| CAS Number | 148-24-3 |
|---|---|
| Molecular Formula | C9H7NO |
| Molecular Weight | 145.161 |
| InChI Key | MCJGNVYPOGVAJF-UHFFFAOYSA-N |
| LogP | 2.02 |
| Synonyms |
|
Applications:
HPLC Method for Analysis of 8-hydroxyquinoline with Primesep 100
April 30, 2018
HPLC Method for 8-Hydroxyquinoline on Primesep 100 by SIELC Technologies
 High Performance Liquid Chromatography (HPLC) Method for Analysis of 8-hydroxyquinoline with Primesep 100
8-Hydroxyquinoline, or oxine, is a common chelating agent that also serves as an effective antiseptic and antifungal compound. Since it is an effective chelating agent, it is often used in LC applications to determine metal contamination in silica-based columns. Even small amounts of metal ions on the silica surface can cause significant peak distortion with very low peak efficiency and poor peak symmetry.
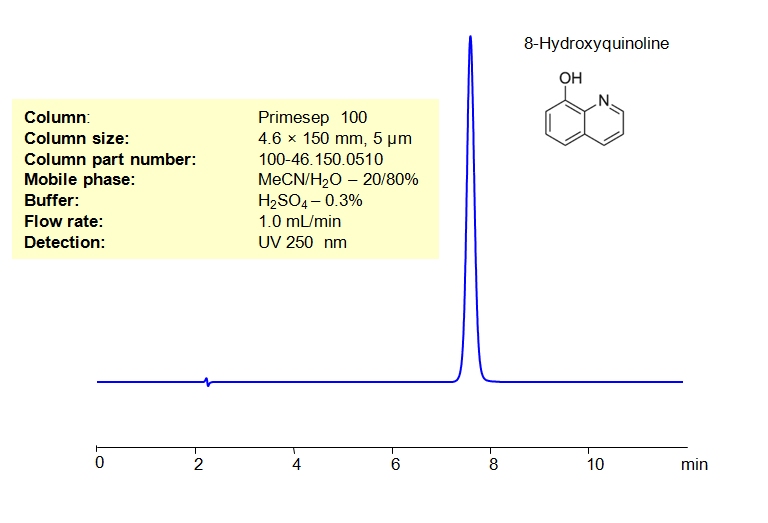
8-Hydroxyquinoline can be retained and analyzed on either a Primesep 100 mixed-mode column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a Sulfuric acid (H2SO4) buffer. Compared to a standard C19 column, Primesep 100 offers significantly better retention characteristics due to the ionic interaction of basic quinoline molecules with the cation-exchange functional groups of the column’s stationary phase.
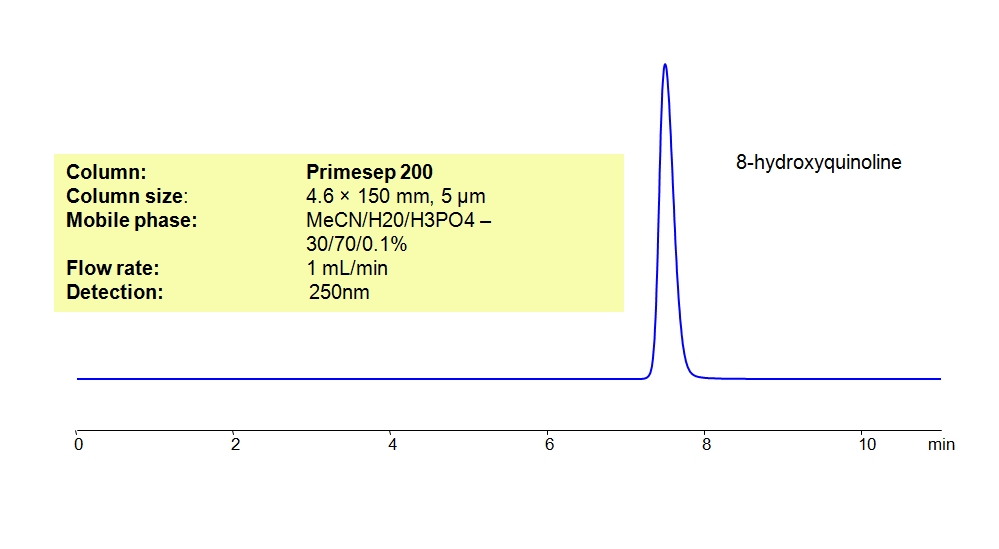
This analytical method can be adapted to a Primesep 200 mixed-mode column by replacing the H2SO4 buffer with Phosphoric acid (H3PO4). The analytical method on either column can be UV detected at 200 nm with high resolution and peak symmetry.
For LC-MS applications, the H3PO4 can be substituted with Ammonium Formate (AmFm) with similar results on a Primesep 200 column.
High Performance Liquid Chromatography (HPLC) Method for Analysis of 8-hydroxyquinoline with Primesep 100
8-Hydroxyquinoline, or oxine, is a common chelating agent that also serves as an effective antiseptic and antifungal compound. Since it is an effective chelating agent, it is often used in LC applications to determine metal contamination in silica-based columns. Even small amounts of metal ions on the silica surface can cause significant peak distortion with very low peak efficiency and poor peak symmetry.
8-Hydroxyquinoline can be retained and analyzed on either a Primesep 100 mixed-mode column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a Sulfuric acid (H2SO4) buffer. Compared to a standard C19 column, Primesep 100 offers significantly better retention characteristics due to the ionic interaction of basic quinoline molecules with the cation-exchange functional groups of the column’s stationary phase.
This analytical method can be adapted to a Primesep 200 mixed-mode column by replacing the H2SO4 buffer with Phosphoric acid (H3PO4). The analytical method on either column can be UV detected at 200 nm with high resolution and peak symmetry.
For LC-MS applications, the H3PO4 can be substituted with Ammonium Formate (AmFm) with similar results on a Primesep 200 column.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 20% |
| Buffer | H2SO4 – 0.3% |
| Flow Rate | 1.0ml/min |
| Detection | UV, 250 nm |
| Class of Compounds | Antiseptic |
| Analyzing Compounds | 8-Hydroxyquinoline |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

HPLC Method for Analysis of 8-hydroxyquinoline with Primesep 200
April 30, 2018
HPLC Method for 8-Hydroxyquinoline on Primesep 200 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of 8-hydroxyquinoline with Primesep 200
8-Hydroxyquinoline, or oxine, is a common chelating agent that also serves as an effective antiseptic and antifungal compound. Since it is an effective chelating agent, it is often used in LC applications to determine metal contamination in silica-based columns. Even small amounts of metal ions on the silica surface can cause significant peak distortion with very low peak efficiency and poor peak symmetry.
8-Hydroxyquinoline can be retained and analyzed on either a Primesep 100 mixed-mode column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a Sulfuric acid (H2SO4) buffer. Compared to a standard C19 column, Primesep 100 offers significantly better retention characteristics due to the ionic interaction of basic quinoline molecules with the cation-exchange functional groups of the column’s stationary phase.
This analytical method can be adapted to a Primesep 200 mixed-mode column by replacing the H2SO4 buffer with Phosphoric acid (H3PO4). The analytical method on either column can be UV detected at 200 nm with high resolution and peak symmetry.
For LC-MS applications, the H3PO4 can be substituted with Ammonium Formate (AmFm) with similar results on a Primesep 200 column.
| Column | Primesep 200, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 25/75% |
| Buffer | H3PO4 – 0.1% |
| Flow Rate | 1.0ml/min |
| Detection | UV, 250 nm |
| Class of Compounds | Antiseptic |
| Analyzing Compounds | 8-Hydroxyquinoline |
Application Column
Primesep 200
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended