| CAS Number | 51-84-3 |
|---|---|
| Molecular Formula | C7H16NO2 |
| Molecular Weight | 146.209 |
| InChI Key | OIPILFWXSMYKGL-UHFFFAOYSA-N |
| LogP | -2.17 |
| Synonyms |
|
Applications:
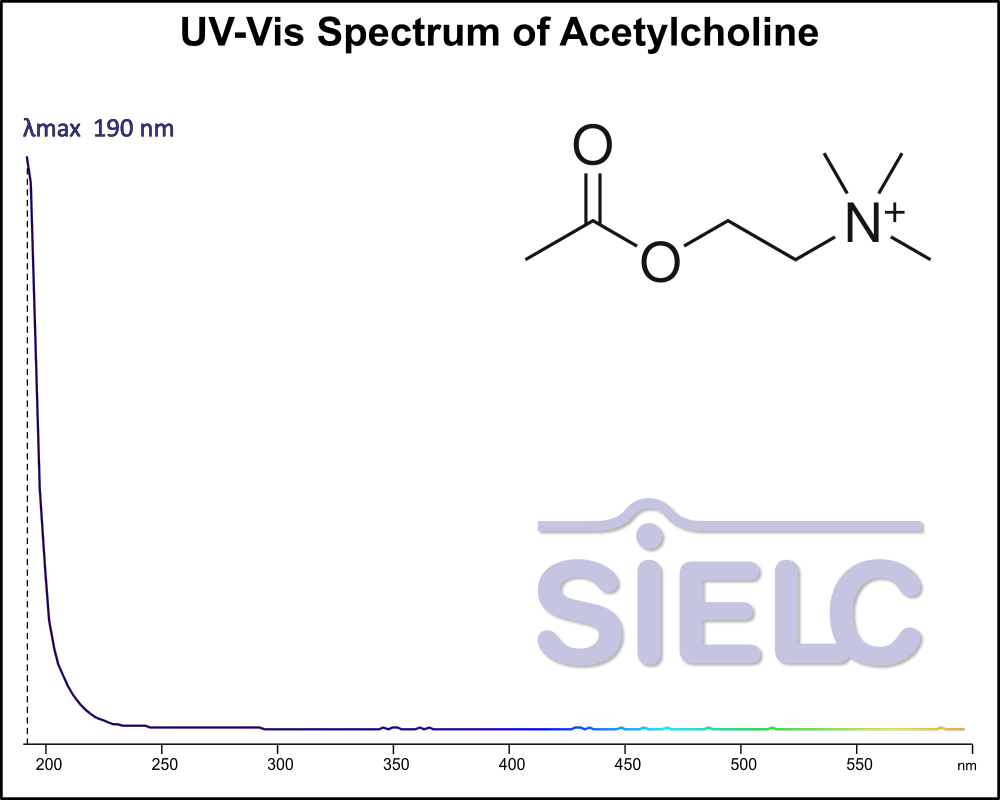
Uv-Vis Spectrum of Acetylcholine
January 22, 2026
If you are looking for optimized HPLC method to analyze Acetylcholine check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

HPLC ELSD Method for Analysis Acetylcholine and Choline on Obelisc R Column
January 18, 2024
HPLC Method for Analysis of Acetylcholine, Choline on Obelisc R by SIELC Technologies
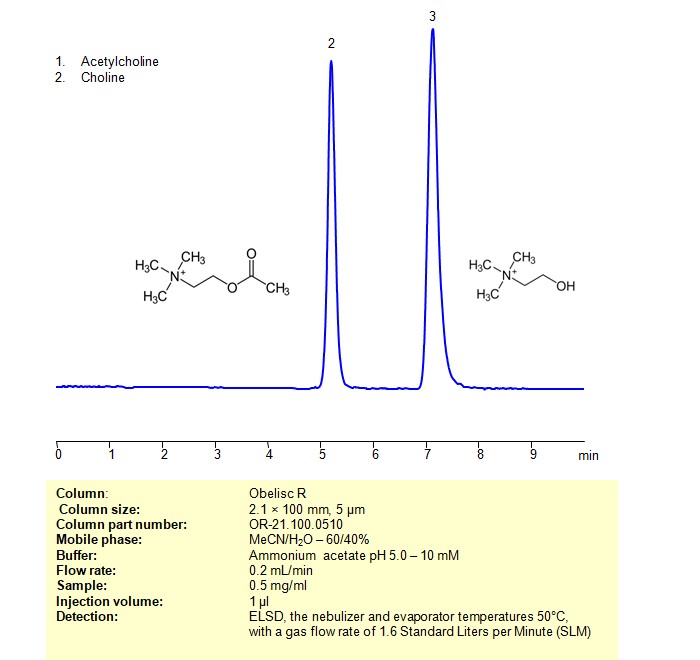
High Performance Liquid Chromatography (HPLC) Method for Analysis of Acetylcholine, Choline
Acetylcholine and choline are related compounds that play distinct roles in the body, particularly in the nervous system.
- Choline:
- Definition: Choline is an essential nutrient that belongs to the B-vitamin complex.
- Chemical Structure: Choline is a quaternary ammonium salt containing a positively charged nitrogen atom and is often written as (N(CH_3)_3^+).
- Function: Choline serves as a precursor for the synthesis of various important molecules, including phospholipids (essential components of cell membranes) and the neurotransmitter acetylcholine. Choline is crucial for maintaining cell membrane integrity and is involved in liver function.
- Dietary Sources: Choline is found in foods such as eggs, meat, fish, nuts, and certain vegetables. It can also be consumed as a dietary supplement.
- Acetylcholine:
- Definition: Acetylcholine is a neurotransmitter, which is a chemical messenger that transmits signals between nerve cells (neurons) or from neurons to muscles.
- Chemical Structure: Acetylcholine is derived from the combination of choline and acetyl coenzyme A. Its chemical formula is (CH_3COOCH_2CH_2N(CH_3)_3^+).
- Function: Acetylcholine plays a crucial role in transmitting signals across synapses, including those between neurons and muscles (neuromuscular junctions). It is involved in muscle contraction, autonomic nervous system functions, and cognitive processes such as memory and learning.
- Synthesis and Release: Acetylcholine is synthesized within nerve cells and released into synapses upon a nerve impulse. Its action is terminated by the enzyme acetylcholinesterase, which breaks it down in the synaptic cleft.
In summary, choline is a nutrient that the body uses to synthesize acetylcholine, among other important molecules. Acetylcholine, on the other hand, is a neurotransmitter responsible for transmitting signals in the nervous system. While choline is obtained from the diet or supplements, acetylcholine is a key mediator of nerve and muscle function in the body.
Acetylcholine and Choline can be retained, separated and analyzed using an Obelisc R mixed-mode stationary phase column. The analysis employs an isocratic method with a simple mobile phase consisting of water, acetonitrile (MeCN), and ammonium acetate as a buffer. Detection is achieved using ELSD
| Column | Obelisc R, 2.1 x 100 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 60/40% |
| Buffer | Ammonium acetate pH 5.0 – 10 mM |
| Flow Rate | 0.2 ml/min |
| Detection | ELSD, the nebulizer and evaporator temperatures 50°C, with a gas flow rate of 1.6 Standard Liters per Minute (SLM) |
| Samples | 0.5 mg/ml |
| Injection volume | 1 µl |
| LOD* | 200 ppb |
| Class of Compounds | Quaternity amines |
| Analyzing Compounds | Acetylcholine, Choline |
Application Column
Obelisc R
Column Diameter: 2.1 mm
Column Length: 100 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Choline

HPLC ELSD Method for Analysis of Acetylcholine on Obelisc R Column
January 15, 2024
HPLC Method for Analysis of Acetylcholine on Obelisc R by SIELC Technologies
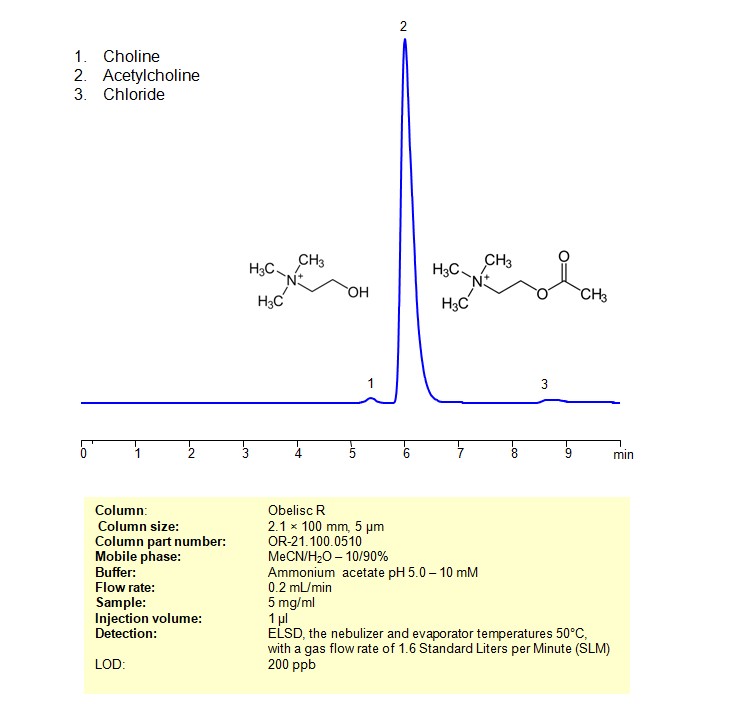
High Performance Liquid Chromatography (HPLC) Method for Analysis of Acetylcholine
Acetylcholine is a neurotransmitter, which is a chemical messenger that transmits signals across synapses, the gaps between nerve cells or between nerve cells and muscles. It plays a crucial role in the nervous system, both in the central nervous system (CNS) and the peripheral nervous system (PNS).
Here are key points about acetylcholine:
- Chemical Structure: Acetylcholine is derived from the combination of choline and acetyl coenzyme A. Its chemical formula is (CH_3COOCH_2CH_2N(CH_3)_3^+).
- Synthesis and Release: Acetylcholine is synthesized within nerve cells through a series of enzymatic reactions. Upon a nerve impulse reaching the end of a nerve cell, acetylcholine is released into the synapse.
- Neuromuscular Junction: In the peripheral nervous system, acetylcholine is the neurotransmitter responsible for transmitting signals from motor neurons to muscle fibers at the neuromuscular junction. The binding of acetylcholine to receptors on the muscle cell membrane triggers muscle contraction.
- Autonomic Nervous System: In the autonomic nervous system, acetylcholine is involved in transmitting signals in the parasympathetic branch. It slows the heart rate, stimulates digestive processes, and promotes a “rest and digest” response.
- Cognitive Functions: In the central nervous system, acetylcholine is implicated in various cognitive functions, including memory, learning, and attention. Its role in the brain is complex and involves interactions with different receptor types.
- Degradation: The enzyme acetylcholinesterase breaks down acetylcholine in the synaptic cleft, terminating its action and preventing continuous stimulation.
- Medicinal Importance: Drugs that affect acetylcholine receptors or inhibit acetylcholinesterase activity are used for various medical purposes. For example, some medications aim to enhance acetylcholine activity in Alzheimer’s disease.
The balance of acetylcholine and other neurotransmitters is essential for proper nervous system function. Imbalances in acetylcholine levels have been implicated in various neurological disorders.
Acetylcholine can be retained and analyzed using an Obelisc R mixed-mode stationary phase column. The analysis employs an isocratic method with a simple mobile phase consisting of water, acetonitrile (MeCN), and ammonium acetate as a buffer. Detection is achieved using ELSD
| Column | Obelisc R, 2.1 x 100 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 10/90% |
| Buffer | Ammonium acetate pH 5.0 – 10 mM |
| Flow Rate | 0.2 ml/min |
| Detection | ELSD, the nebulizer and evaporator temperatures 50°C, with a gas flow rate of 1.6 Standard Liters per Minute (SLM) |
| Samples | 5 mg/ml |
| Injection volume | 1 µl |
| LOD* | 200 ppb |
| Class of Compounds | Quaternity amines |
| Analyzing Compounds | Acetylcholine |
Application Column
Obelisc R
Column Diameter: 2.1 mm
Column Length: 100 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

HPLC Separation of Acetylcholines
May 6, 2004
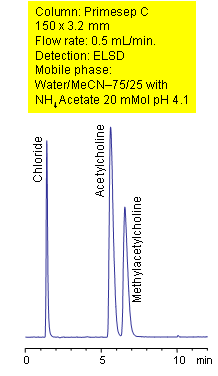
Primesep C separates acetylcholine and methylacetylcholine from their chloride counter ion with baseline resolution by a combination of cation exchange, complex formation, and hydrophobic interactions. The mass spec (LC/MS) compatible method uses a mobile phase of water, acetonitrile (MeCN, ACN) and ammonium acetate with evaporative light scattering detection (ELSD).
| Column | Primesep C, 3.2×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc ph 4.1 |
| Flow Rate0.5 | ml/min |
| Detection | ELSD |
| Class of Compounds |
Drug |
| Analyzing Compounds | Acetylcholine, Methylacetylcholine |
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsMethylacetylcholine