| CAS Number | 132-22-9 |
|---|---|
| Molecular Formula | C16H19ClN2 |
| Molecular Weight | 274.791 |
| InChI Key | SOYKEARSMXGVTM-UHFFFAOYSA-N |
| LogP | 3.38 |
| Synonyms |
|
Applications:
HPLC Separation of Drugs in Tylenol Cold and Cough Remedies
August 22, 2008
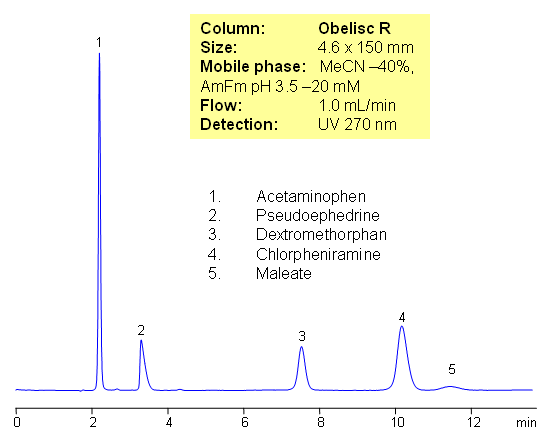
Components of Tylenol Cold and Cough Remedy are separated on Obelisc R mixed-mode column. Method can be used to determine compounds in various cough and cold compositions. Neutral (acetaminophen), basic (chlorpheniramine, dextromethorphan and pseudoephedrine) and acidic (maleic acid/Maleate) components are analyzed with perfect peak shape and retention control. Method can be used in production, QC/QA and biological studies for quantitation of various components in pharmaceutical formulations (Advil, Tylenol, Dimetapp, Robitussin, NyQuil, etc)
| Column | Obelisc R, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN |
| Buffer | AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Analgetic, Acid, Hydrophilic, Ionizable, Neutral, Basic |
| Analyzing Compounds | Maleate, Chlorpheniramine, Dextromethorphan, Pseudoephedrine, Acetaminophen |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsChlorpheniramine
Dextromethorphan
Maleate
Pseudoephedrine (PSE)