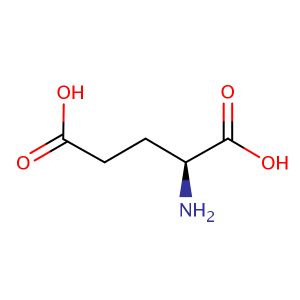
| CAS Number | 56-86-0 |
|---|---|
| Molecular Formula | C5H9NO4 |
| Molecular Weight | 147.13 |
| InChI Key | WHUUTDBJXJRKMK-VKHMYHEASA-N |
| LogP | -2.77 |
| Synonyms |
|
Applications:
HPLC Method for Separation of Ibotenic Acid and Glutamic Acid on Primesep A Column
February 28, 2023
HPLC Method for Separation of Ibotenic Acid and Glutamic Acid on Primesep A by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
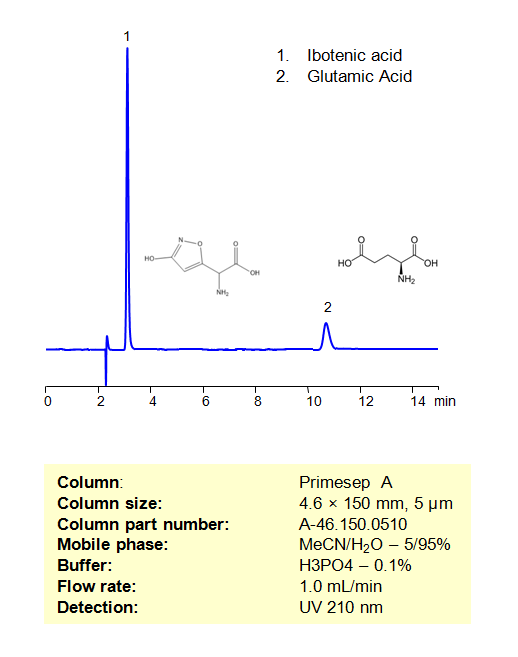
Ibotenic acid is a physchoactive NDMA and metabotropic glutamate receptor agonist with neurotoxic properties. These structurally similar psychoactive drugs can be retained, separated, and analyzed on a mixed-mode Primesep A column with a mobile phase consisting of water, Acetonitrile (MeCN), and Phosphoric acid (H3PO4). This analytical method can be UV detected at 210 nm with high resolution and peak symmetry.
High Performance Liquid Chromatography (HPLC) Method for Analysis of Ibotenic Acid and Glutamic Acid
Condition
| Column | Primesep A, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 5/95% |
| Buffer | H3PO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 210 nm |
| Peak Retention Time | 3.22 min, 10.52 min |
Description
| Class of Compounds | Acid |
| Analyzing Compounds | Ibotenic Acid, Glutamic Acid |
Application Column
Primesep A
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
GLU (L-Glutamic acid)
Glutamic Acid
Ibotenic acid
L-Glutamic acid hydrochloride

HPLC Separation of a Mixture of Non-Essential Amino Acids, such as L-Aspartic Acid, L-Serine, L-Glutamic Acid, and L-Alanine on Primesep 100 Column
March 11, 2019
High Performance Liquid Chromatography (HPLC) Method for Analysis of Non-Essential Amino Acids on Primesep 100 by SIELC Technologies
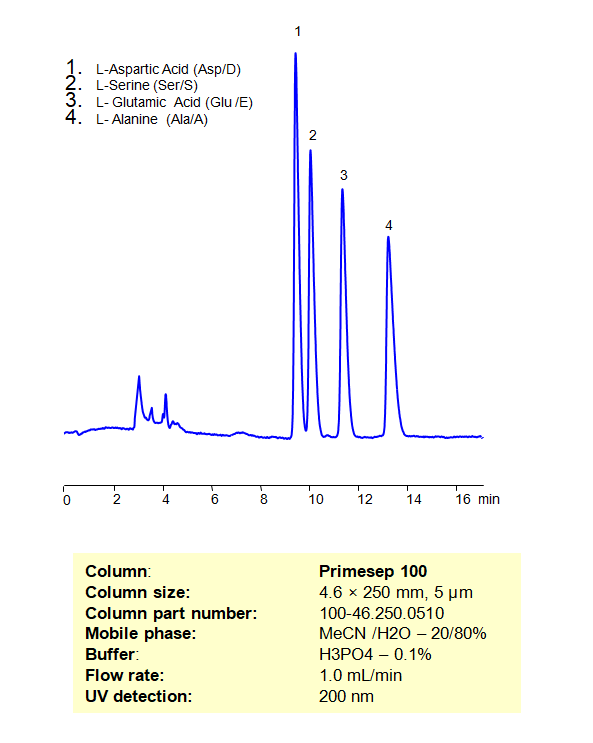
High Performance Liquid Chromatography (HPLC) Method for Analyses of Non-Essential Amino Acids
| Column | Primesep 100, 4.6 x 250 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 20/80% |
| Buffer | H3PO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds | Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Aspartic Acid, Serine, GLU (L-Glutamic acid), Glutamic Acid, D-Alanine, DL-Alanine, Amino Acids, Alanine |
Amino acids are the building blocks of proteins. Based on their dietary requirement, they are classified into essential and non-essential amino acids. Essential amino acids cannot be synthesized by the human body in sufficient quantities and must be obtained from the diet. Non-essential amino acids, on the other hand, can be synthesized by the body and are not dependent on dietary intake.
It’s worth noting that while these amino acids are considered “non-essential” for adults under normal circumstances because the body can synthesize them, there are situations where some may become “conditionally essential.” This means that under certain conditions like illness, stress, or trauma, the body might not produce them in sufficient quantities, and dietary intake becomes necessary. Arginine, for instance, is considered conditionally essential, especially during periods of rapid growth, illness, or trauma.
Amino acids can be retained, separeted and analyzed on a Primesep 100 mixed-mode stationary phase column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a phosphoric acid (H3PO4) as a buffer. This analysis method can be detected in the UV regime at 200 nm.
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 250 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Amino Acids
Aspartic Acid
D-Alanine
DL-Alanine
GLU (L-Glutamic acid)
Glutamic Acid
Serine

HPLC Separation of GABA and GLU
August 22, 2008
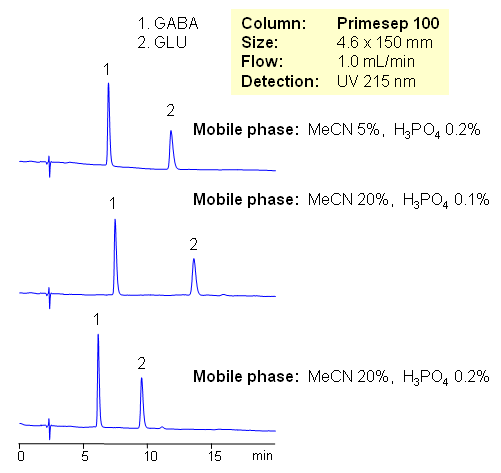
Amino acids are building blocks for peptides and proteins; they are used in various supplement formulations and as starting reagents in chemistry to introduce chirality. GABA and GLU are used to enhance growth of specified plants, prevent development of powdery mildew on grapes, and suppress certain other plant diseases. L-Glutamic acid is one of the major amino acids naturally found in plant and animal proteins, and GABA helps to maintain normal brain function. Both amino acids behave as neurotransmitters. All amino acids have both basic and acidic groups. Depending on pH amino acids can be basic, acidic or zwitter-ionic. Due to the polar nature of underivatized amino acids, analysis is a very challenging task. Derivatization and ion-pairing reagents are used to provide retention of amino acids. Simple method is developed on Primesep 100 column using combination of acetonitrile/water with phosphoric acid as a mobile phase. Method uses UV detection. Amino acids are well retained without use of ion-pairing reagents. Fast reliable method can be developed for all underivatized natural and synthetic amino acids.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H3PO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 215 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | GABA, Glu |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsgamma-Aminobutyric Acid (GABA)