| CAS Number | 1320-04-3 |
|---|---|
| Molecular Formula | C11H8O2 |
| Molecular Weight | 172.183 |
| InChI Key | LNETULKMXZVUST-UHFFFAOYSA-N |
| LogP | 3.1 |
| Synonyms |
|
Applications:
HPLC Separation of Organics Acids
November 21, 2006
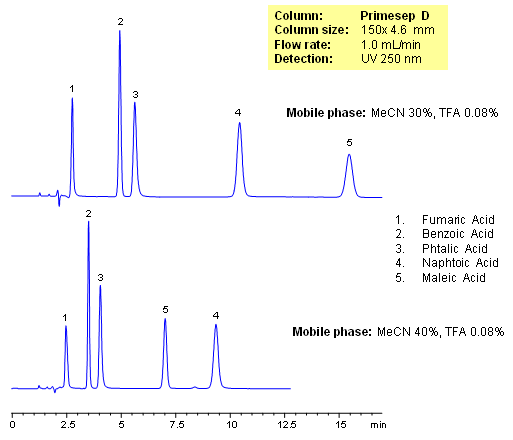
Primesep D separates organic acids such as fumaric, benzoic, phthalic, naphthoic, and maleic acids by a mixture of anion exchange and reversed phase. Retention times and elution order can be changed by adjusting the percentage of acetonitrile in the mobile. This can not be done by traditional ion-exchange and ion-exclusion chromatography. The HPLC separation uses a mobile phase of water, acetonitrile (MeCN, ACN) and trifluoroacetic acid (TFA) and UV detection at 250 nm.
| Column | Primesep D, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | UV 250 nm |
| Class of Compounds |
Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Fumaric Acid, Benzoic Acid, Phthalic Acid, Maleic Acid, Naphtoic Acid |
Application Column
Primesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsFumaric Acid
Maleic Acid
Naphthoic Acid
Organic Acids
Phthalic Acid

Separation of Diacid Hydrophobic and Ion Exchange Modes
October 11, 2005
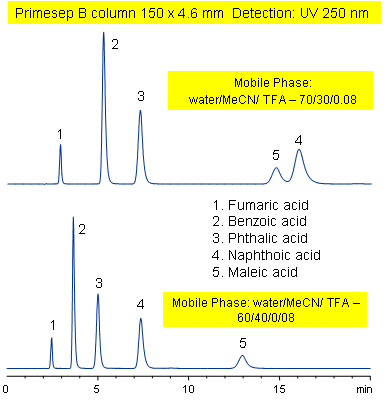
Primesep B combines a hydrophobic, reversed-phase mechanism with ion exchange to separate the diacids, fumaric, benzoic, phthalic, naphthoic, and maleic acids. Changing the acetonitrile content of the mobile phase reverses the peak order for naphthoic and maleic acids. Primesep B combines reversed-phase and anion-exchange mechanism with a mobile phase of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) and UV detection at 250 nm.
| Column | Primesep B, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | TFA |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Fumaric acid, Benzoic acid, Phthalic acid, Naphthoic acid, Maleic acid, ) |
Application Column
Primesep B
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDicarboxylic Acids
Fumaric Acid
Maleic Acid
Naphthoic Acid
Phthalic Acid