| CAS Number | 10549-76-5 |
|---|---|
| Molecular Formula | C16H36N |
| Molecular Weight | 242.470 |
| InChI Key | DZLFLBLQUQXARW-UHFFFAOYSA-N |
| LogP | 1.04 |
| Synonyms |
|
Applications:
Separation of Sodium, Tetramethylammonium and Tetrabutylammonium on Primesep 200 Column
July 7, 2011
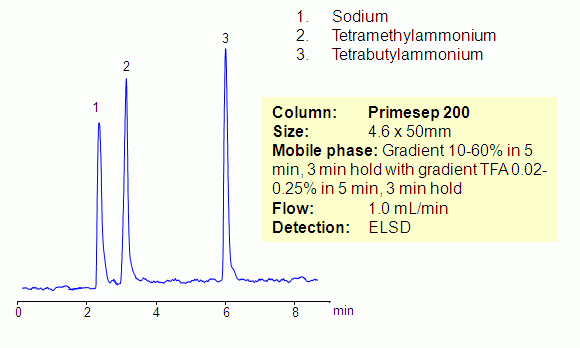
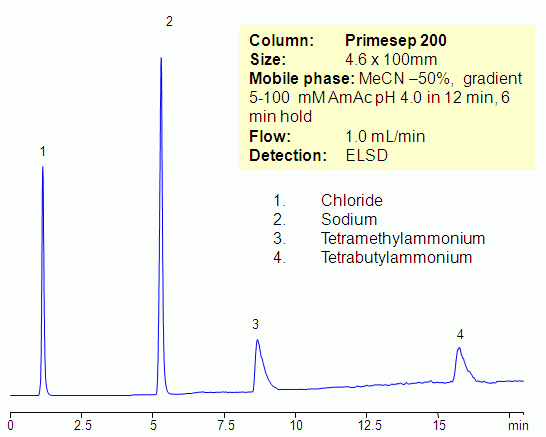
Hydrophilic and hydrophobic quaternary amines, along with sodium ion, were separated by mixed-mode chromatography on a Primesep 200 column. Mechanism of retention for sodium and tetramethylammonium ions is cation exchange, while the tetrabutylammonium ion is retained by combination of reversed-phase and cation-exchange mechanisms. All three compounds are not UV-active and monitoring is done by ELSD/CAD.
| Column | Primesep 200, 4.6×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc pH4.0 |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Ions, Hydrophilic, Ionizable, Quaternary amines |
| Analyzing Compounds | Sodium, Chloride, Tetramethylammonium, Tetrabutylammonium |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsTetrabutylammonium
Tetramethylammonium

Separation of Quaternary Amines by Reverse-Phase Mechanism
January 16, 2004
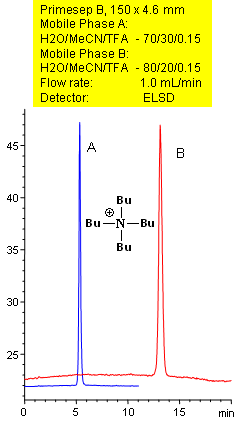
Primesep B separates quaternary amines, such as t-butylamine with symmetrical peak shape by a combination of reversed-phase and ion-exclusion mechanisms. The embedded basic functional group on the stationary phase shields the underlying silanols to prevent peak tailing. Excellent peak shape results with a mass spec compatible mobile phase of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) with evaporative light scattering detection (ELSD).
| Column | Primesep B, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | TFA |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Ions, Hydrophilic, Ionizable, Quaternary amines |
| Analyzing Compounds | t-butylamine |
Application Column
Primesep B
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options