| CAS Number | 60-54-8 |
|---|---|
| Molecular Formula | C22H24N2O8 |
| Molecular Weight | 444.440 |
| InChI Key | OFVLGDICTFRJMM-WESIUVDSSA-N |
| LogP | -1.30 |
| Synonyms |
|
Applications:
Uv-Vis Spectrum of Tetracycline
January 23, 2026

If you are looking for optimized HPLC method to analyze Tetracycline hydrochloride, Tetracycline check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.
Tetracycline hydrochloride

HPLC Method for Analysis of Tetracycline on Primesep 100 Column on Alltesta™
September 29, 2025
HPLC Method for Tetracycline on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Tetracycline
Tetracycline is an antibiotic with the molecular formula C22H24N2O8. It is a bacteriostatic agent, therefore it prevents bacteria from growing and multiplying through inhibiting protein synthesis within bacteria. It is used to treat a variety of infections.
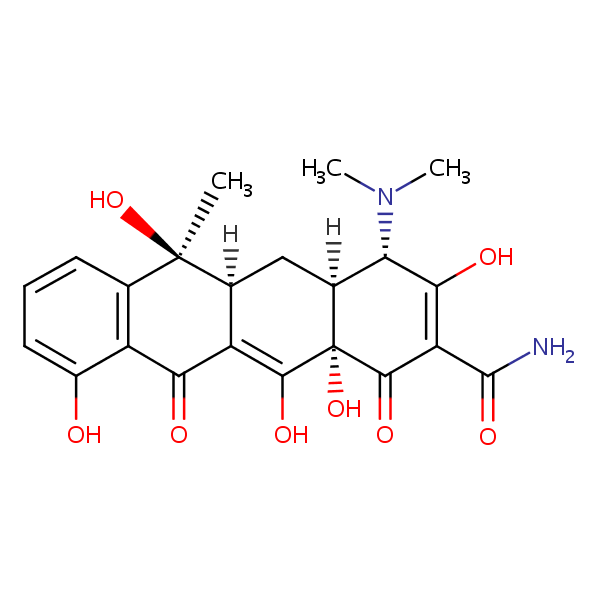
Tetracycline can be retained and analyzed using the Primesep 100 stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a sulfuric acid buffer. Detection is performed using UV.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 20-80% |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 275 nm |
| Class of Compounds | Medication |
| Analyzing Compounds | Tetracycline |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended

HPLC Method For Analysis Of Tetracycline on Primesep 100 Column
August 9, 2021
HPLC Method for Tetracycline hydrochloride, Tetracycline on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Tetracycline
Tetracycline is a broad spectrum antibiotic, that is, it is active against many different types of bacteria. It is effective against Chlamydia psittaci, Neisseria gonorrhoeae, Haemophilus influenzae, Streptococcus pneumoniae, Mycoplasma pneumoniae, Chlamydia trachomatis, and others. Tetracycline prevents the growth of bacteria by preventing the bacteria from manufacturing proteins they need to survive.
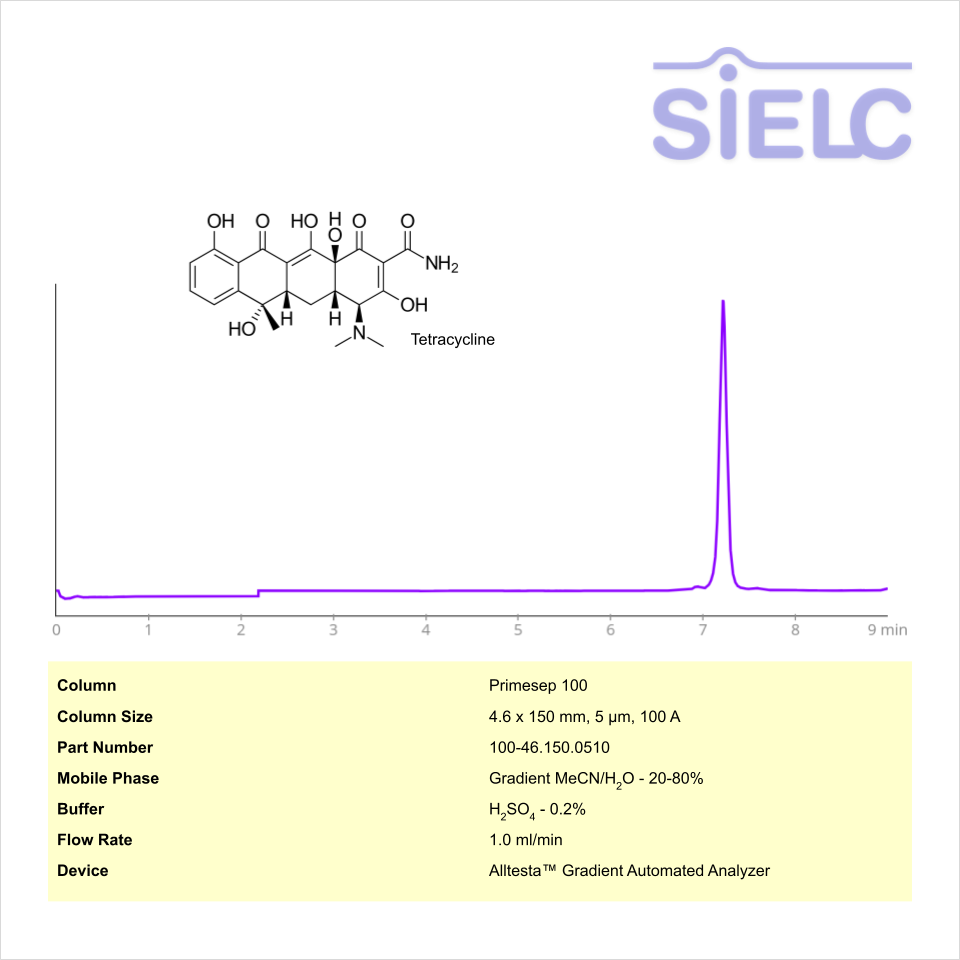
Tetracycline can be retained on the Primesep 100 mixed-mode column using an isocratic analytical method with a simple mobile phase of water, acetonitrile (MeCN, ACN), and sulphuric acid (H2SO4) buffer. The analysis method can be UV detected at 200 nm.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 50/50% |
| Buffer | H2SO4 – 0.5% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds |
Drug, Antibiotics |
| Analyzing Compounds | Tetracycline hydrochloride, Tetracycline |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Tetracycline hydrochloride

Separation of Antibiotics in Mixed-mode chromatography
May 11, 2015
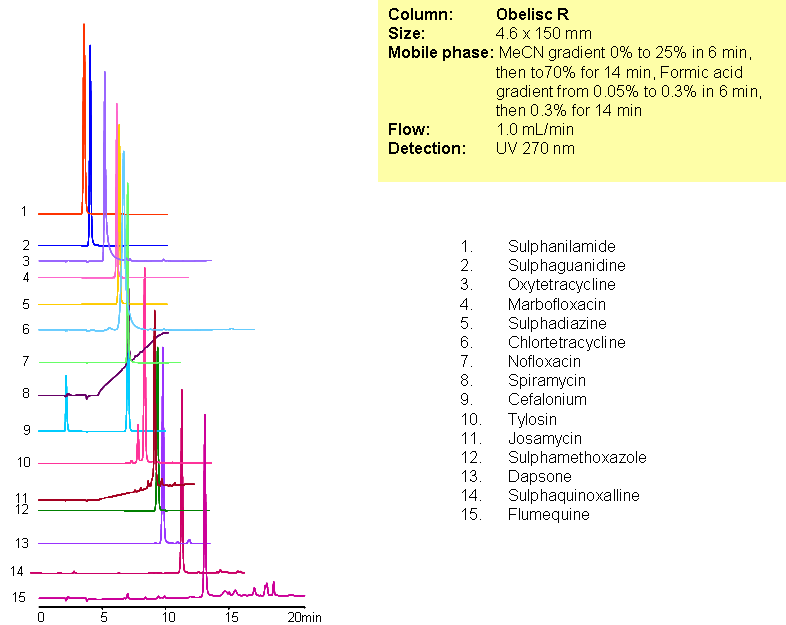
A complex mixture of sulphonamide, macrolide, tetracycline and fluoroquinolone antibiotics were separated in one run using mixed-mode chromatography with LC/MS -compatible conditions. All compounds are separated based on reversed-phase and/or ion-exchange mechanism. Method can be used for analysis of various classes of antibiotics and related impurities in different sample matrices (blood, urine, soil, waste water).
| Column | Obelisc R, 2.1×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 0-25%, 6 min, 25-70% 14 min |
| Buffer | Gradient Formic Acid – 0.05%-0.3%, 10 min, 14 min hold |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Antibiotic, Drug, Hydrophobic, Ionizable |
| Analyzing Compounds | Sulphanilamide, Sulphaguanidine, Oxytetracycline, Marbofloxacin, Sulphadiazine, Chlortetracycline, Nofloxacin, Spiramycin, Cefalonium, Tylosin, Josamycin, Sulphamethoxazole, Dapsone, Sulphaquinoxalline, Flumequine |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsChlortetracycline
Dapsone
Flumequine
Josamycin
Marbofloxacin
Norfloxacin
Oxytetracycline
Spiramycin
Sulfamethoxazole
Sulfonamides
Sulphadiazine
Sulphaguanidine
Sulphanilamide
Sulphaquinoxaline
Tetracycline
Tylosin
UV Detection

HPLC Separation of Tetracyclines
November 21, 2010
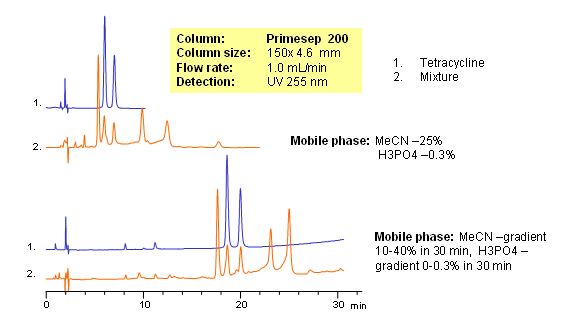
Tetracyclines are a group of broad spectrum of antibiotics. Structure can be described as derivatives of polycyclic naphthacene carboxamide. Tetracyclines are hydrophilic and basic compounds due to the presence of hydroxyls and amino group. Mixture of tetracyclines and their impurities were separated based on weak reversed-phase and strong cation-exchange mechanisms on a Primesep 200 mixed-mode HPLC column. Elution of compounds was monitored by UV.
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options