| CAS Number | 52-53-9 |
|---|---|
| Molecular Formula | C27H38N2O4 |
| Molecular Weight | 454.611 |
| InChI Key | SGTNSNPWRIOYBX-UHFFFAOYSA-N |
| LogP | 3.79 |
| Synonyms |
|
Applications:
HPLC Method for Analysis of Verapamil
May 6, 2016
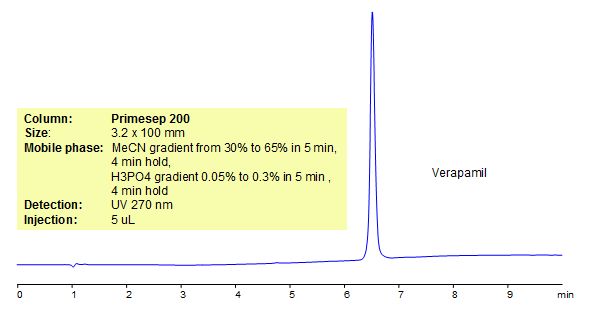
Verapamil is a phenylalkylamine calcium channel blocker that is a class IV anti-arrhythmia agent. It is used to treat high blood pressure, as well as hypertension, angina pectoris and superventricular tachyarrhythmias. The drug also helps prevent strokes, heart attacks, and kidney problems. Primesep 200, a reverse phase column, contains embedded acidic ionizable groups and can retain Verapamil. The method is UV compatible and can be used as a general approach for analyzing similar compounds.
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsVerapamil hydrochloride

HPLC Analysis of Basic Drugs and Acidic Counter-Ions by Mixed-Mode Chromatography
July 16, 2009
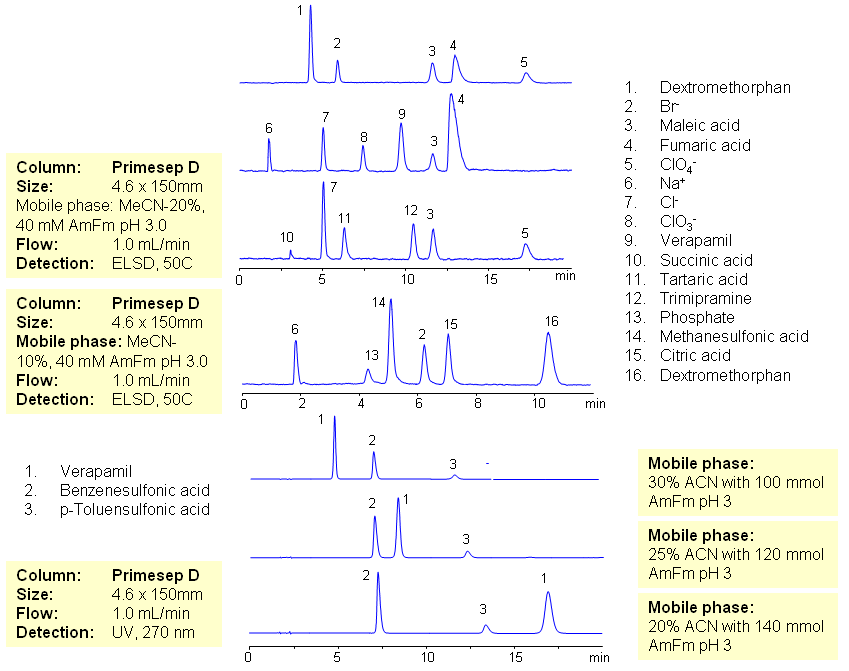
The majority of drugs in the pharmaceutical industry are administered in salt form. The presence of two counter-ions very often necessitates the use of two methods. The nature of these counterparts in drugs can be an inorganic cation and organic acid, inorganic anion and organic base, and organic cation and organic anion. Furthermore, the properties of the molecules will result in a differing stoichiometry. The task of simultaneous quantitation of counter-ions can be achieved by using mixed-mode columns. The general approach for analysis is based on properties of corresponding counter-ions. Hydrophobic basic drugs, like dextromethorphan, verapamil, trimipramine, and corresponding acidic counter-ions (chloride, chlorate, bromide, bromate, perchlorate, maleate, fumarate,tartrate, succinate, phosphate, citrate, benzosulfonate, toleuensulfonate) can be separated and quantitated in the same run on reversed-phase anion-exchange column. Basic hydrophobic drugs are retained by the reversed-phase mechanism, and counter-ions are retained by the reversed-phase and anion-exchange mechanism. Some polar counter-ions are retained only by the anion-exchange mechanism. Retention time and selectivity of HPLC separation of drugs and counter-ions can be achieved by changing the amount of acetonitrile and the amount of ions in the mobile phase. The detection technique depends on the properties of the counter-ions. In case of low or no UV activity, ELSD can be employed if the counter-ion forms a non-volatile salt with the mobile phase additive (ammonium formate). This HPLC method can be used for simultaneous quantitation of other basic drugs and counter-ions. The presence of two mechanisms of retention allows control over retention times of drug and counter-ion independently, and even allows a change of order of elution when necessary.
| Column | Primesep D , 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm pH 3.0 |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD, UV 270 |
| Class of Compounds | Ions, Hydrophilic, Hydrophobic, Base, Acids, Ionizable |
| Analyzing Compounds | Sodium Chloride, Sodium chloride, Sodium Chlorate, Sodium bromide, Sodium bromate, Perchloric Acid, Maleic Acid, Fumaric Acid, Tartaric Acid, Succinic Acid, Phosphoric Acid, Citric acid, Benzosulfonic acid, Dextromethorphan, Verapamil, Trimipramine |
Application Column
Primesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsBromide
Chlorate
Chloride
Citric Acid
Dextromethorphan
Fumaric Acid
Maleic Acid
Organic Acids
Perchlorate
Phosphoric Acid
Pyrilamine
Succinic Acid
Tartaric Acid
Verapamil
p-Toluenesulfonic Acid (PTSA)
UV Detection