| CAS Number | 61-19-8 |
|---|---|
| Molecular Formula | C10H14N5O7P |
| Molecular Weight | 347.224 |
| InChI Key | UDMBCSSLTHHNCD-KQYNXXCUSA-N |
| LogP | -1.78 |
| Synonyms |
|
Applications:
HPLC Separation of Adenosine mono-, di- and triphosphates on Newcrom BH Column
May 11, 2021
Separation type: Liquid Chromatography Mixed-mode
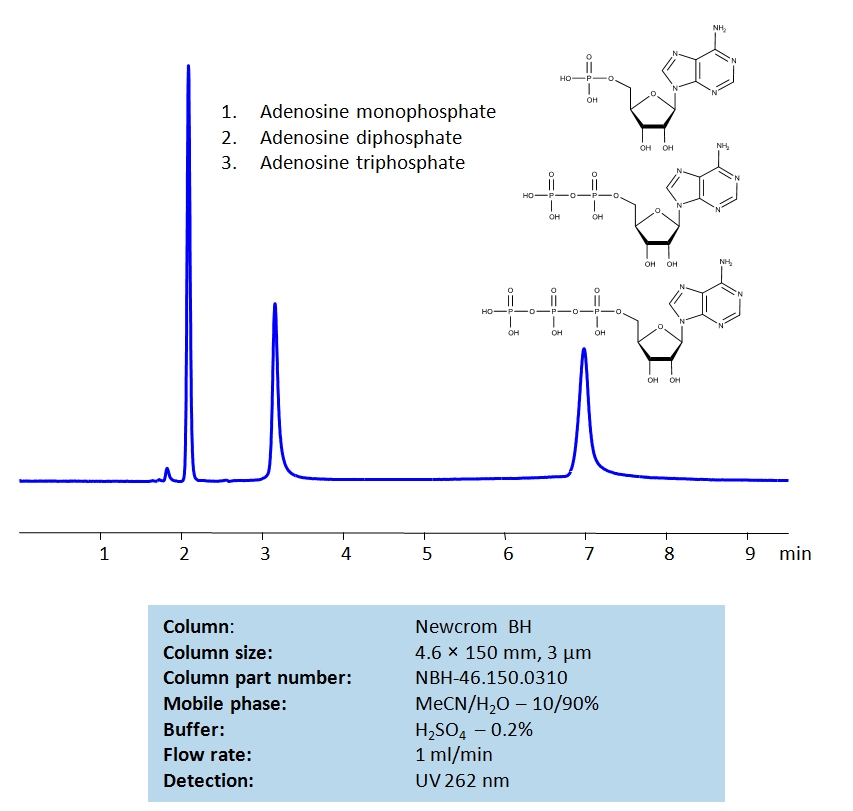
High Performance Liquid Chromatography (HPLC) Method for Analysis of Adenosine monophosphate (AMP), Adenosine diphosphate (ADP), Adenosine triphosphate (ATP)
Adenosine mono-, di- and triphosphates are key components of the cell’s energy ecosystem. Adenosine triphosphate (ATP) is the main unit of energy used in the cell. When it is ‘spent,’ it is converted to adenosine diphosphate (ADP); it is often converted back to ATP to be reused. Adenosine monophosphate can be converted to ADP (and subsequently ATP), but it is also a key factor in RNA synthesis.
Using a Newcrom B mixed-mode column and a mobile phase consisting of water and acetonitrile with a sulfuric acid (H2SO4) buffer, adenosine mono-, di- and triphosphate can be separated, measured, and analyzed. This method can UV detect this family of compounds at 262 nm with very high resolution.
| Column | Newcrom BH, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 10/90% |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 262nm |
| Class of Compounds | Acid, Hydrophilic |
| Analyzing Compounds | Adenosine monophosphate (AMP) Adenosine diphosphate (ADP) Adenosine triphosphate (ATP) |
Application Column
Newcrom B
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsNewcrom BH
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsAdenosine Monophosphate
Adenosine Triphosphate

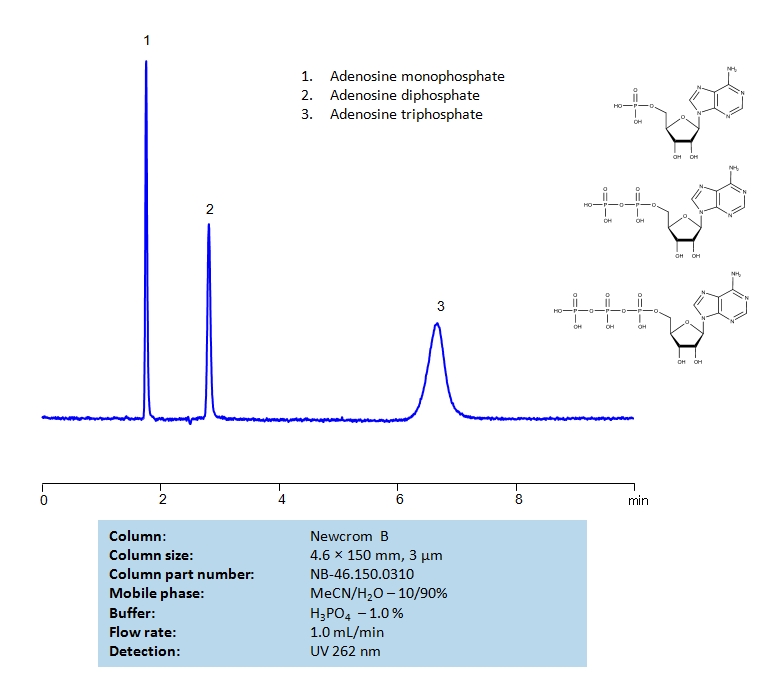
HPLC Separation of Adenosine Mono-, Di- and Triphosphate on Newcrom B column
April 13, 2020
| Column | Newcrom B, 4.6×150 mm, 3 µm, 100A |
| Mobile Phase | MeCN/H2O – 10/90% |
| Buffer | H2SO4 – 1.0% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 262nm |
| Class of Compounds | Acid, Hydrophilic |
| Analyzing Compounds | Adenosine monophosphate (AMP) Adenosine diphosphate (ADP) Adenosine triphosphate (ATP) |
Application Column
Newcrom B
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsAdenosine Monophosphate
Adenosine Triphosphate

Separation of Nucleotide Monophosphates
July 13, 2015
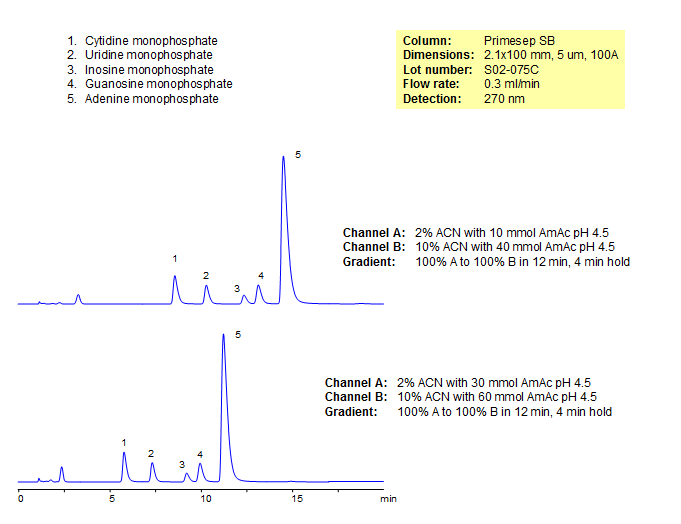
Nucleotides are the monomers of DNA and RNA, composed of a five-carbon sugar, a nitrogenous base, and at least one phosphate group. Primesep SB is a good column for separating these highly polar compounds. Primesep SB is a reverse-phase column with strong embedded basic ion-pairing groups. Retention can me manipulated by adjusting acetonitrile, and the baseline separation can be achieved in under 6 minutes using a gradient.
Application Column
Primesep SB
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsCytidine Monophosphate
Guanosine Monophosphate
Inosine Monophosphate
Uridine Monophosphate

Separation of Nine Nucleotides by Mixed-Mode Chromatography
July 6, 2015
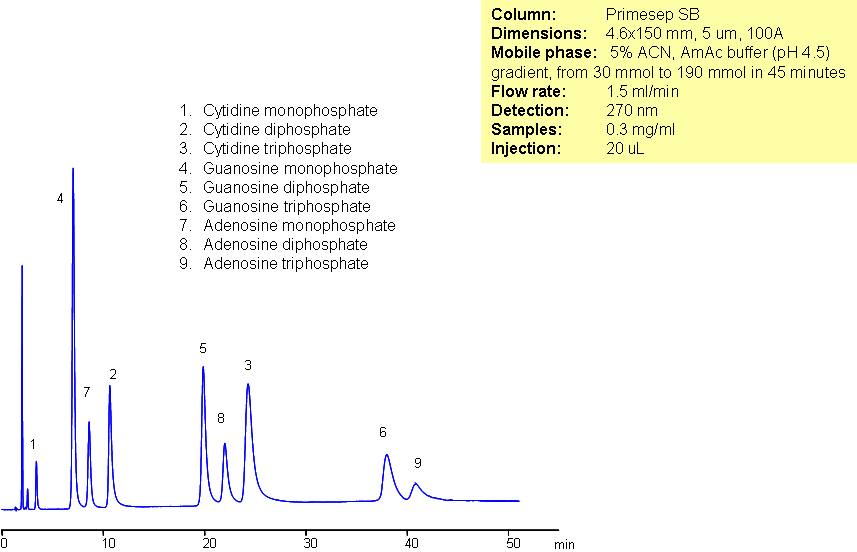
Nucleotides are important biological molecules which serve as subunits of nucleic acids. They are composed of a five-carbon sugar, a nitrogenous base, and at least one phosphate group. Nucleotides cannot be retained by reverse-phase chromatography without an ion-pairing reagent due to their highly polar nature. Primesep SB is capable of retaining and separating nine nucleotides. Primesep SB is a reverse-phase column with strong embedded basic ion-pairing groups.
Application Column
Primesep SB
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAdenosine Monophosphate
Adenosine Triphosphate
Cytidine Diphosphate
Cytidine Monophosphate
Cytidine Triphosphate
Guanosine Diphosphate
Guanosine Monophosphate
Guanosine Triphosphate

Separation of Nicotinamide and Related Substances
May 11, 2015
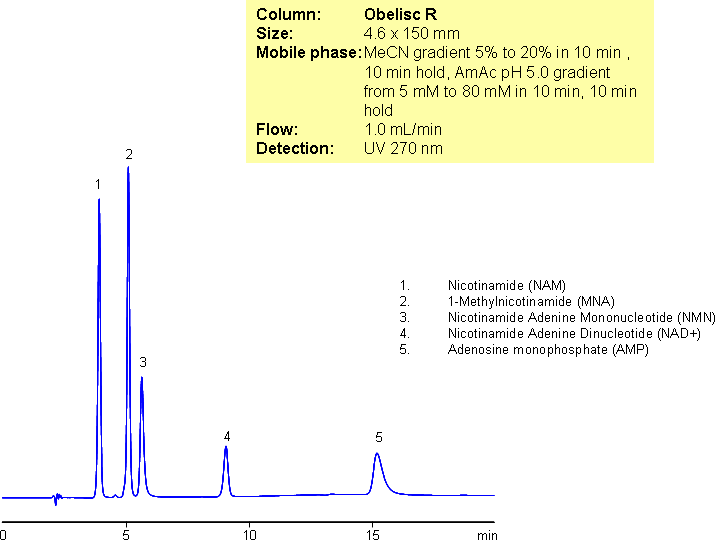
A complex mixture of nicotinamide and related impurities was separated on Obelisc R mixed-mode column. Nicotinamide, methylnicotinamide, nicotinamide adenine mononucleotide, nicotinamide adenine dinucleotide, and adenosine monophosphate were baseline resolved in a 15 minute long method. This mixed-mode approach can be used for analysis of other nucleotides. Obelisc R trimodal column separates this complex mixture based on reversed-phase, cation-exchange and anion-exchange mechanisms. Retention is controlled by amount of ACN, buffer concentration and buffer pH. Additional selectivity can be gained by exploring various buffers within the same pH
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsAdenosine Monophosphate
Nicotinamide
Nicotinamide Adenine Dinucleotide (NAD)
Nicotinamide Adenine Mononucleotide
UV Detection

HPLC Separation of Adenosine Mono-, Di- and Triphosphate in Reversed-Phase Mixed-Mode with LC/MS Compatible Conditions
December 5, 2013
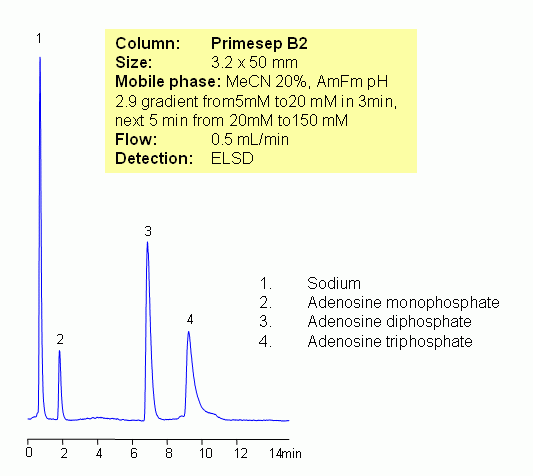
Adenosine mono-, di and triphosphate are hydrophilic nucleotides which serve as building blocks of DNA and RNA. Each molecule consists of phosphate or phosphate groups, adenine and sugar ribose. Molecules are hydrophilic and lack a retention mechanism on traditional reversed-phase column. Three nucleotides were retained and separated on Primesep B2 reversed-phase anion-exchange column. retention time is controlled by buffer concentration and buffer pH. ADP and ATP require higher concentration of buffer to facilitate elution. Method can be used for LC/MS analysis of different nucleotides in various sample matrices (biofluids, plasma, blood, urine). Other detection techniques can be used for analysis. Method is reliable and robust and can tolerate interference from sample matrix. Additional sample preparation might be required.
| Column | Primesep B2, 3.2×50 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 20/80% |
| Buffer | AmFm pH 2.9- 5-20 mM 3 min, 20-150 mM |
| Flow Rate | 0.5 ml/min |
| Detection | ELSD |
| Class of Compounds |
Nucleotide, Hydrophilic, Ionizable |
| Analyzing Compounds | Adenosine Monophosphate, Adenosine Diphosphate, Adenosine Triphosphate |
Application Column
Primesep B2
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAdenosine Monophosphate
Adenosine Triphosphate