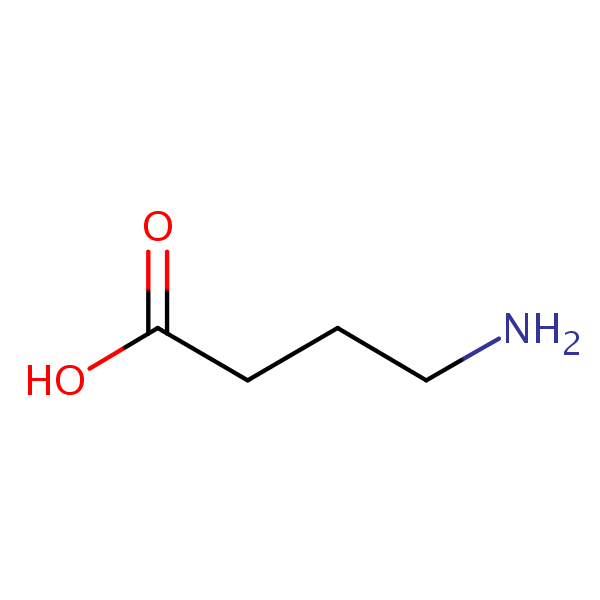
| CAS Number | 56-12-2 |
|---|---|
| Molecular Formula | C4H9NO2 |
| Molecular Weight | 103.122 |
| InChI Key | BTCSSZJGUNDROE-UHFFFAOYSA-N |
| LogP | -3.17 |
| Synonyms |
|
Applications:
HPLC Method for Determination of Gamma-aminobutyric acid (GABA), Vigabatrin, Phenibut, Pregabalin, Baclofen on Primesep 100 Column
June 15, 2021
Separation type: Liquid Chromatography Mixed-mode
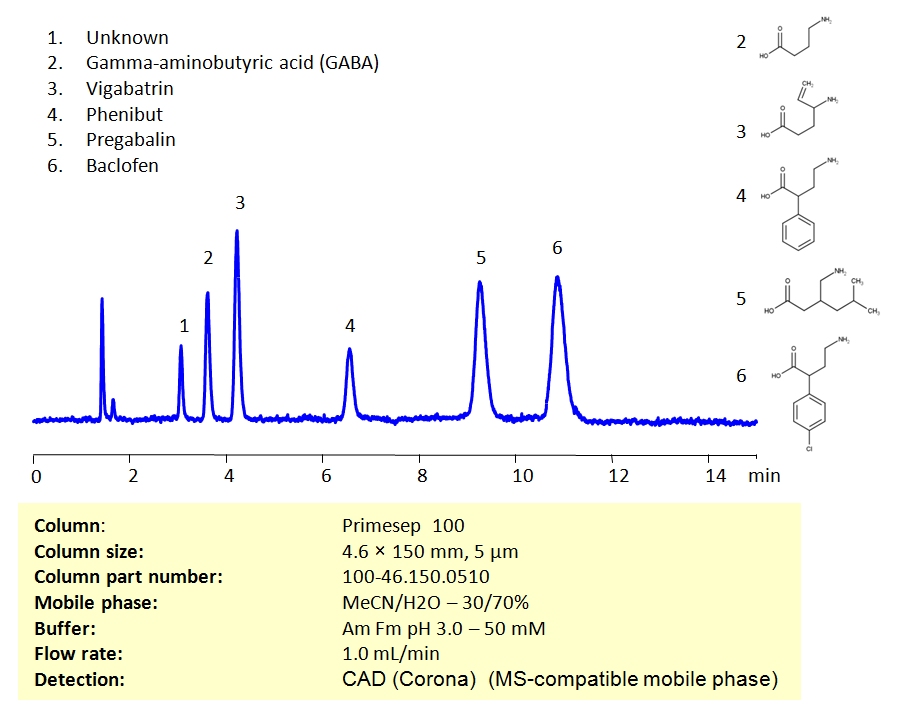
High Performance Liquid Chromatography (HPLC) Method for Analysis of Gamma-aminobutyric acid (GABA), Vigabatrin, Phenibut, Pregabalin, Baclofen
All gabapentinoids can be retained and separated on the Primesep 100 mixed-mode column using an isocratic analytical method with a simple mobile phase of water, acetonitrile (MeCN, ACN), and Ammonium Formate (AmFm) buffer. Detection can be achieved with mass spectrometry (MS), evaporative light scattering detection (ELSD) and Charged aerosol detection (CAD).
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | AmFm pH 3.0 – 50 mM |
| Flow Rate | 1.0 ml/min |
| Detection | CAD (Corona) (MS-compatible mobile phase) |
| Class of Compounds | Acid, Drug |
| Analyzing Compounds | Gamma-aminobutyric acid (GABA), Vigabatrin, Phenibut, Pregabalin, Baclofen |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPhenibut
Pregabalin
Vigabatrin
gamma-Aminobutyric Acid (GABA)

HPLC Determination of Gamma-aminobutyric Acid (GABA), Gabapentin and Pregabalin on Primesep N Column
June 8, 2021
Separation type: Liquid Chromatography Mixed-mode
![]()
View on hplc.cloud
High Performance Liquid Chromatography (HPLC) Method for Analysis of GABA, Gabapentin and Pregabalin
| Column | Primesep N, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm pH 3.0 – 10 mM |
| Flow Rate | 1.0 ml/min |
| Detection | CAD (Corona) (MS-compatible mobile phase) |
| Class of Compounds |
Acid, Drug |
| Analyzing Compounds | Gamma-Aminobutyric acid (GABA), Gabapentin, Pregabalin |
Application Column
Primesep N
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsGabapentin hydrochloride
Pregabalin
gamma-Aminobutyric Acid (GABA)

HPLC Separation of Glutamic Acid and GABA
July 8, 2011
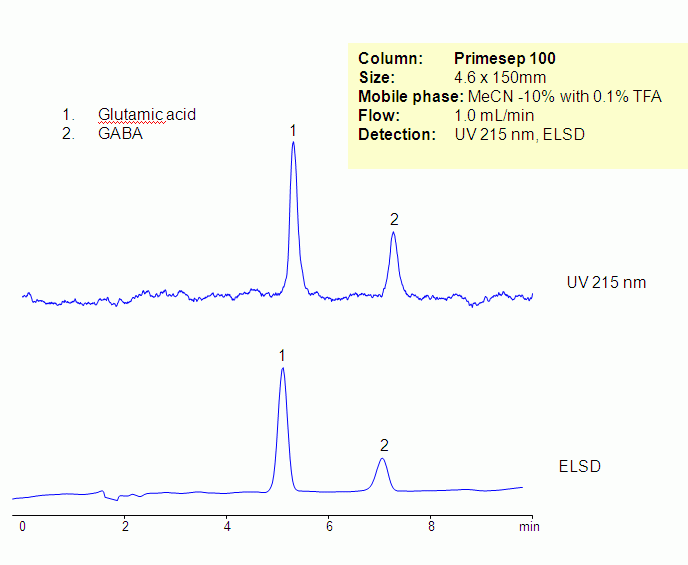
Glutamic acid and GABA are neurotransmitters. Glutamic acid and GABA are non-essential amino acids. They are hydrophilic and zwitter-ionic in nature . At lower pH, carboxylic acid groups of amino acids are not ionized, making them more hydrophobic and basic. Underivatized glutamic acid and GABA were retained and separated on a Primesep 100 column using ACN/water/TFA mobile phase. Amino acids can be monitored by low UV or ELSD/CAD. Retention is provided by reversed-phase and cation-exchange mechanism. Method can be used for analysis of underivatized amino acids in various matrices including supplements, vitamin and other complex mixtures. various mobile phase can be used with corresponding detection techniques.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 10/90% |
| Buffer | TFA – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 215 nm, ELSD |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Glutamic acid, GABA |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsZwitterion
gamma-Aminobutyric Acid (GABA)
UV Detection

HPLC Method for Separation of Serine, a-Methylserine, GABA, and Methionine Mixture on Primesep 100 column
November 21, 2010
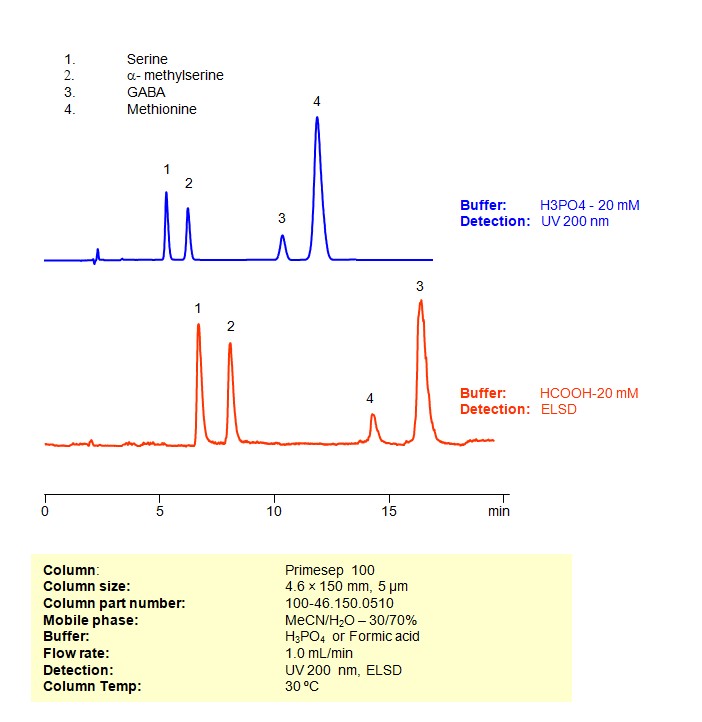
Four underivatized amino acids (serine, methylserine, GABA and methionine) were separated on a Primesep 100 reversed-phase cation-exchange mixed-mode HPLC column. Two methods, one for UV and one for ELSD/LC/MS show good separation and peak shape for underivatized amino acids. This column and general HPLC approach can be used for analysis of underivatized amino acids. Primesep 100 is designed to replace reversed-phase HPLC column in combination with ion-pairing reagents.
| Column | Primesep 100, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H3PO4 or Formic acid |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm, ELSD |
| Class of Compounds | Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Serine, Methylserine, GABA, Methionine |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Serine
a-Methylserine
gamma-Aminobutyric Acid (GABA)

HPLC Separation of alpha-Aminobutyric, beta-Aminobutyric, and gamma-Aminobutyric acids on Obelisc N
March 3, 2010
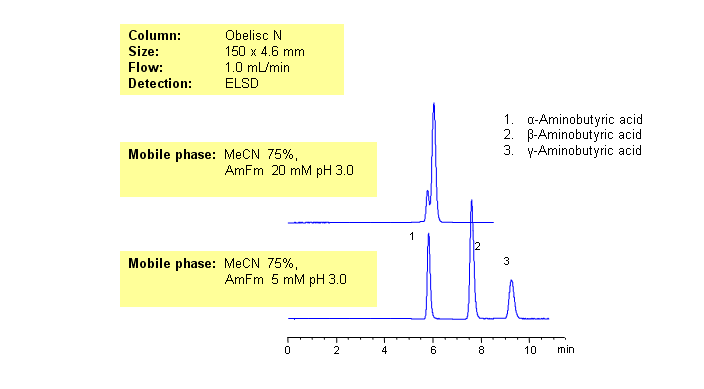
GABA (neurotransmitter) and its isomers are polar zwitter-ionic compounds. Due to the position of amino-groups, all three compounds show different polar and basic properties. The isomers of aminobuturic acid are separated on an Obelisc N HILIC/cation-exchange column. Buffer concentration has a different effect on retention of alpha-, beta-, and gamma-aminobutyric acid. This general and robust method can be used for separation of other polar and ionizable compounds and isomers by mixed-mode chromatography.
| Column | Obelisc N, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm pH 3.0 |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Acid |
| Analyzing Compounds | Alpha-Aminobutyric acid, Beta-Aminobutryic acid, Gamma-Aminobutyric acid (GABA) |
Application Column
Obelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsalpha-Aminobutyric Acid
beta-Aminobutyric Acid
gamma-Aminobutyric Acid (GABA)

HPLC Separation of Polar Compounds
January 13, 2010
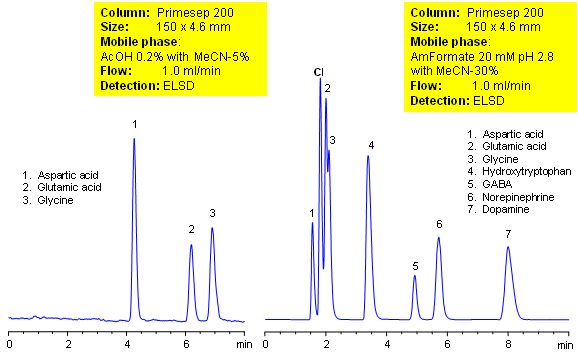
The separation of amino acids, the building blocks of proteins, can be challenging to separate on a reverse-phase column due to their high polarity. Using a mixed-mode HPLC column, allows the separation of amino acids by cation-exchange and ion-exclusion mechanisms as well as hydrophobicity. Fine tuning of separation can be achieved with changes in organic concentration of the mobile phase as well as choice of buffer and pH.
| Column | Primesep 200, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AcOH, AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Aspartic acid, Glutaric acid, Glycine, Hydroxytriptophan, GABA, Norepinephrine, Dopamine |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAspartic Acid
Dopamine
Glutamic Acid
Norepinephrine
gamma-Aminobutyric Acid (GABA)

HPLC Separation of Amino Acids in Supplements Composition in Mixed-Mode
July 16, 2009
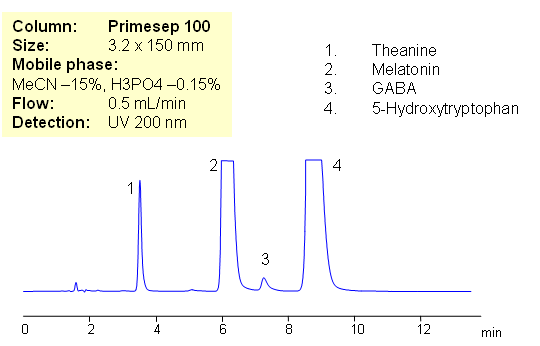
Amino acids are essential components of numerous formulation. Health supplements can contain various amino acids and vitamins and require quantitation of each ingredients. Amino acids are very polar compounds with limited or no retention in reversed-phase chromatography. The most common approaches are reversed-phase chromatography with ion-pairing reagent and hydrophilic interaction chromatography (HILIC). Underivatized amino acids can be retained by combination of reversed-phase and cation exchange mechanism on Primesep 100 mixed-mode. Retention time is controlled by amount of acetonitrile, buffer and buffer pH. Method does not require ion-pairing reagent. This method is for UV detection. LC/MS, ELSD or Corona CAD can be employed for analysis of amino acids with trifluoroacteic acid or ammonium formate in the mobile phase. This approach can be used for HPLC analysis of all underivatized amino acids.
| Column | Primesep 100, 3.2×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 15/85% |
| Buffer | H3PO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Theanine, Melatonin, GABA, 5- Hydroxytryptophan |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAmino Acids
Melatonin
Theanine
gamma-Aminobutyric Acid (GABA)

HPLC Separation of GABA and GLU
August 22, 2008
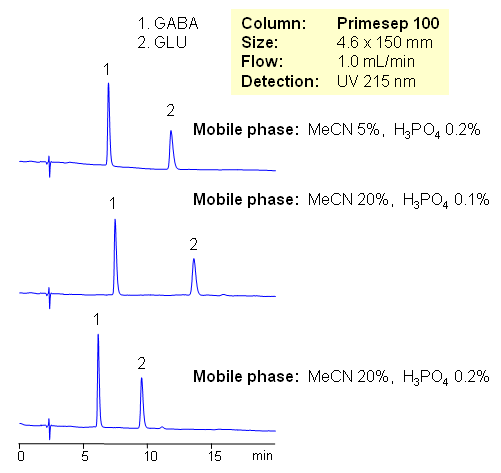
Amino acids are building blocks for peptides and proteins; they are used in various supplement formulations and as starting reagents in chemistry to introduce chirality. GABA and GLU are used to enhance growth of specified plants, prevent development of powdery mildew on grapes, and suppress certain other plant diseases. L-Glutamic acid is one of the major amino acids naturally found in plant and animal proteins, and GABA helps to maintain normal brain function. Both amino acids behave as neurotransmitters. All amino acids have both basic and acidic groups. Depending on pH amino acids can be basic, acidic or zwitter-ionic. Due to the polar nature of underivatized amino acids, analysis is a very challenging task. Derivatization and ion-pairing reagents are used to provide retention of amino acids. Simple method is developed on Primesep 100 column using combination of acetonitrile/water with phosphoric acid as a mobile phase. Method uses UV detection. Amino acids are well retained without use of ion-pairing reagents. Fast reliable method can be developed for all underivatized natural and synthetic amino acids.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H3PO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 215 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | GABA, Glu |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsgamma-Aminobutyric Acid (GABA)

HPLC Separation of Amino Acids
December 6, 2007

Amino acids are building blocks for peptides and proteins. Serine is not essential to human diet and it is synthesized in human body. Methionine is not synthesized in human body and needs to be ingested. GABA is used to enhance growth of specified plants, prevent development of powdery mildew on grapes, and suppress certain other plant diseases. In humans GABA helps to maintain normal brain function. Serine, methylserine, GABA and methionine are separated on Primesep 100 column by mixed-mode mechanism. Amino acids are retained by combination of reverse phase and cation-exchange mechanisms. At lower pH carboxylic acid fragment of amino acid is suppressed and not ionized, making amino acids more basic and slightly more hydrophobic. Amount of acetonitrile, buffer concentration and buffer pH can be used to adjust retention time. Underivatized amino acids are well retained and separated with perfect peak shape and symmetry. Fast method can be used for UV, ESLD and LC/MS quantitation of serine, methylserine, GABA and methionine.
| Column | Primesep 100, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H3PO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm, ELSD |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Serine, Methylserine, GABA, Methionine |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsSerine
a-Methylserine
gamma-Aminobutyric Acid (GABA)
UV Detection

HPLC Separation of Isomers of Aminobutyric Acids
May 6, 2004
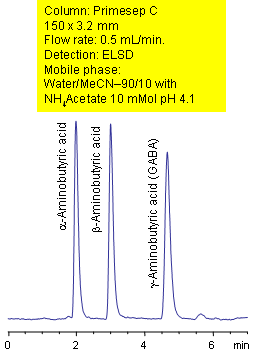
Primesep C separates the isomers of aminobutyric acids by a combination of reversed-phase and ionic interaction mechanisms. alpha-Aminobutyric acid, beta-Aminobutryic acid, and gamma-Aminobutyric acid (GABA) are baseline resolved without ion-pair reagents. The HPLC separation uses a mobile phase of water, acetonitrile (MeCN, ACN) ammonium acetate with evaporative light scattering detection (ELSD).
| Column | Primesep C, 3.2×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc pH 4.1 |
| Flow Rate | 0.5 ml/min |
| Detection | ELSD |
| Class of Compounds |
Acid |
| Analyzing Compounds | Alpha-Aminobutyric acid, Beta-Aminobutryic acid, Gamma-Aminobutyric acid (GABA) |
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsalpha-Aminobutyric Acid
beta-Aminobutyric Acid
gamma-Aminobutyric Acid (GABA)