| CAS Number | 75-75-2 |
|---|---|
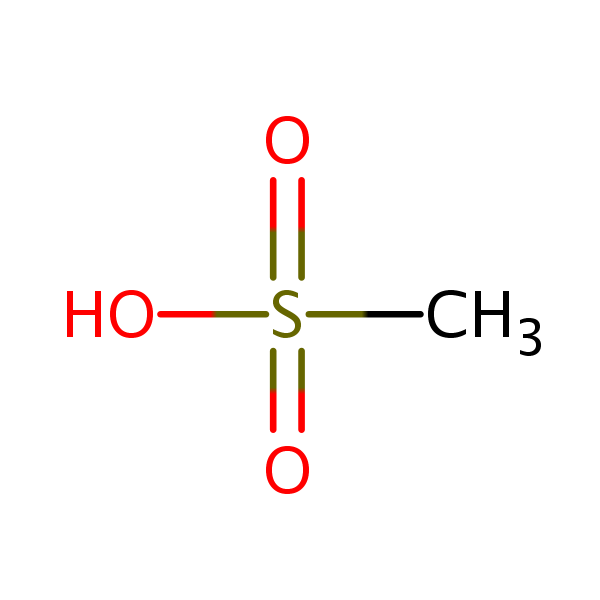
| Molecular Formula | CH4O3S |
| Molecular Weight | 96.100 |
| InChI Key | AFVFQIVMOAPDHO-UHFFFAOYSA-N |
| LogP | -1.61 |
| Synonyms |
|
Applications:
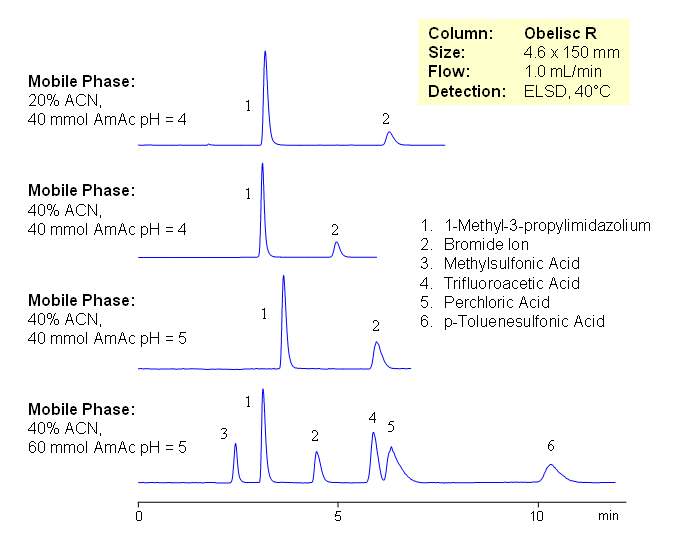
HPLC Analysis of Components of Ionic Liquids by Mixed-Mode Chromatography
September 14, 2008
Ionic liquid is an ionic compound which is liquid at room (or close to room) temperature. Most of the ionic liquids are in a dynamic equilibrium where at any time more than 99.99% of the liquid is made up of ionic, rather than molecular, species. Room-temperature ionic liquids consist of bulky cation (for example, substituted imidazolium) compounds. A wide range of anions is used as counter ions in ionic liquids: organic and inorganic anions such as chloride, iodide, tetrafluoroborate, hexafluorophosphate, bistriflimide, triflate, tosylate. Ionic liquids are widely used as solvents in organic reactions. When products are isolated from ionic liquids, they need to be analyzed for residual ionic liquid content.
Because both constituents of the ionic liquid are very different in terms of charge and hydrophobic properties, it is impossible to analyze entire ionic liquids by traditional chromatography. An effective and universal method for analysis of ionic liquids is developed on an Obelisc R HPLC column. Components on the ionic liquids are retained based on ionic and hydrophobic interactions. Obelisc R column has both positively and negatively charged ionic groups, making it possible to retain and separate cations and anions of ionic liquids on one column. Method can be used for quantitative of various ionic liquids containing organic and inorganic ions. Retention time of basic component can be effectively adjusted by pH, stronger anionic and hydrophobic counter-ions might require higher buffer concentration. Composition can be monitored by combination of UV and ELSD or by LC/MS.
| Column | Obelisc R , 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Acid |
| Analyzing Compounds | 1-Methyl-3-propylimidazolium, Bromide Ion, Methylsulfonic Acid, Trifluoroacetic Acid, Perchloric Acid, p-Toluenesulfonic Acid |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsBromide
Ionic Liquid
Methylsulfonic Acid
Perchloric Acid
TFA (Trifluoroacetic Acid)
p-Toluenesulfonic Acid (PTSA)
UV Detection