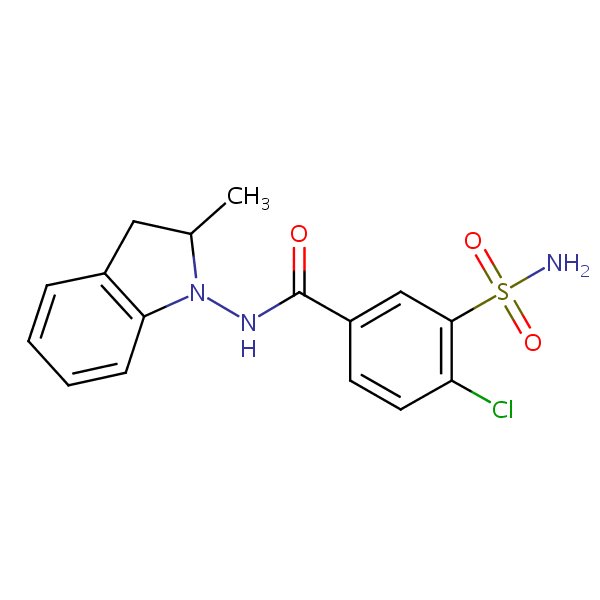
| CAS Number | 26807-65-8 |
|---|---|
| Molecular Formula | C16H16ClN3O3S |
| Molecular Weight | 365.830 |
| InChI Key | NDDAHWYSQHTHNT-UHFFFAOYSA-N |
| LogP | 1.95 |
| Synonyms |
|
Applications:
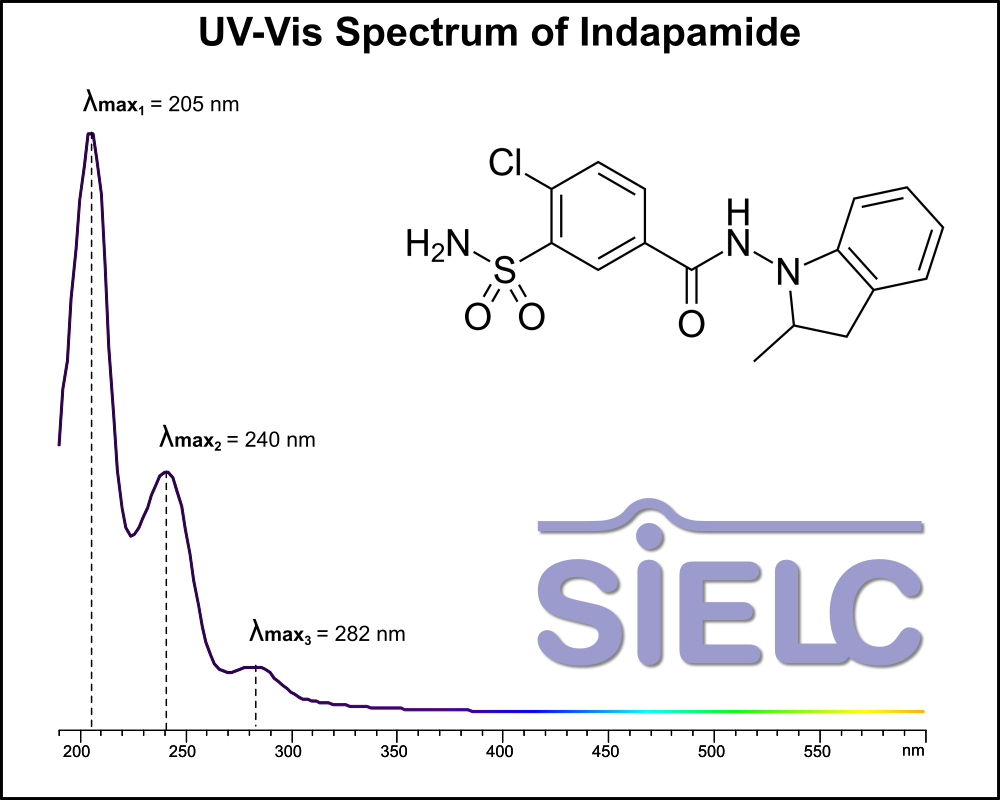
Uv-Vis Spectrum of Indapamide
February 16, 2026
Access the UV-Vis Spectrum SIELC Library
If you are looking for optimized HPLC method to analyze Indapamide check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

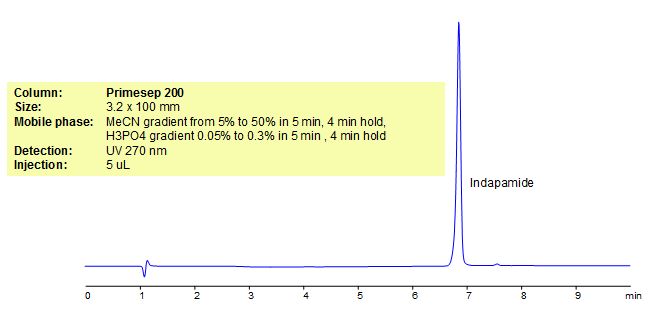
HPLC Method for Analysis of Indapamide
April 29, 2016
Indapamide is an indole derivative of chlorosulphonamide and a sulfamyl diuretic with antihypertensive activity. It exerts its diuretic effect by inhibiting reabsorption of sodium and chloride, primarily as a result of action on the cortical diluting segment of the renal distal tubule. Indapamide is also used in the treatment of hypertension. Primesep 200, a reverse phase column, contains embedded acidic ionizable groups and can retain Indapamide. The method is UV compatible and can be used as a general approach for analyzing similar compounds.
| Column | Primesep 200, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 5-50% |
| Buffer | Gradient H3PO4 – 0.05-0.3%, 5 min, 4 min hold |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, |
| Analyzing Compounds | Indapamide |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options