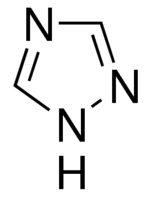
| Molecular Formula | C2H3N3 |
|---|---|
| Molecular Weight | 69.07 |
| InChI Key | NSPMIYGKQJPBQR-UHFFFAOYSA-N |
| LogP | -0.6 |
| Synonyms |
|
Applications:
HPLC Method for Separation of 1,2,4-Triazole and Guanylurea on Primesep 100 Column
January 9, 2024
HPLC Method for Analysis of Guanylurea, 1,2,4-Triazole on Primesep 100 by SIELC Technologies
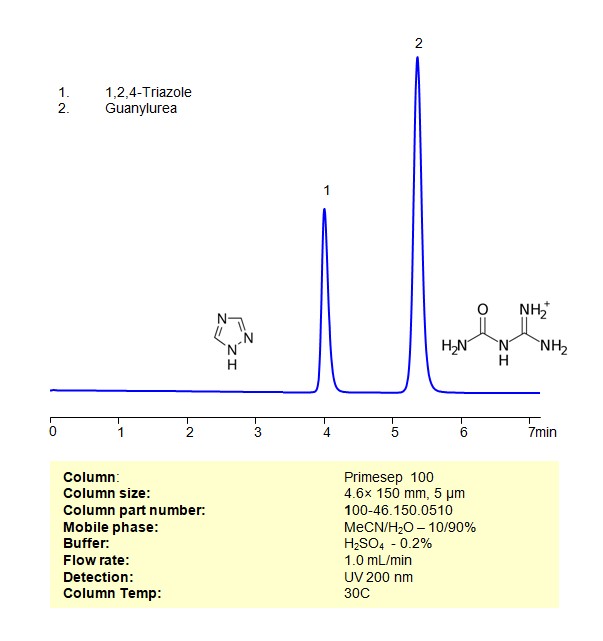
High Performance Liquid Chromatography (HPLC) Method for Analysis of Guanylurea, 1,2,4-Triazole
Guanylurea, also known as guanidylurea, is a chemical compound with the molecular formula (C_3H_7N_5O). It is derived from guanidine and urea, and it has interesting applications in the field of chemistry and materials science.
- Crystal Growth: Guanylurea is known for its use in crystal growth processes. It has been utilized as an additive in solution to control and promote the growth of certain crystals, including organic and inorganic crystals. Guanylurea is part of the broader field of crystal engineering, where researchers explore the design and synthesis of crystalline materials with specific properties. The ability of guanylurea to form hydrogen bonds makes it relevant in this context.
- Hydrogen Bonding Studies: Due to its unique structure, guanylurea has been studied for its hydrogen bonding properties, which are important in various chemical and biological processes.The presence of amino groups in guanylurea allows it to participate in hydrogen bonding interactions, which are crucial in determining the structure and properties of molecular assemblies.
It’s important to note that guanylurea’s applications are primarily in the realm of crystal engineering and related studies. Its ability to participate in hydrogen bonding interactions makes it a valuable component in the design and manipulation of crystalline materials for various purposes.
1,2,4-triazole is a heterocyclic compound with a unique five-membered ring structure. Its versatility, biological activities, and ability to coordinate with metal ions make it a compound of interest in various scientific and medicinal applications.
The 1,2,4-triazole and Guanylurea be retained, separated and analyzed using a Primesep 100 mixed-mode stationary phase column. The analysis employs an isocratic method with a simple mobile phase comprising water, acetonitrile (MeCN), and sulfuric acid as a buffer. This method allows for detection using UV at 200 nm
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A |
| Mobile Phase | MeCN/H2O – 10/90% |
| Buffer | H2SO4 -0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 nm |
| Samples | Guanylurea – 0.77mg/mL 1,2,4-Triazole – 3.9mg/mL |
| Injection volume | 3 µl |
| LOD* | Guanylurea – 5 ppb 1,2,4-Triazole – 25 ppb |
| Class of Compounds | Aromatic amines, Ureas |
| Analyzing Compounds | Guanylurea, 1,2,4-Triazole |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Guanylurea

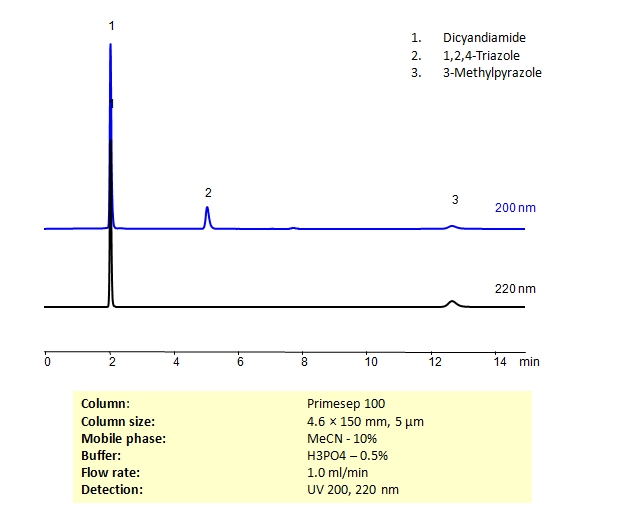
HPLC Separation of Dicyandiamide, 1,2,4-Triazole, 3-Methylpyrazole on Primesep 100 Column
December 20, 2019
Dicyandiamide, or cyanoguanidine, is used as a curing agent for epoxy resins. 1,2,4-triazole, is a heterocycle used primarily as an antifungal but has other uses in the pharmaceutical industry as well. 3-methylpyrazole is used in nitrogen fertilizers. All three compounds are structurally similar and can be separated in HPLC using Primesep 100 reverse-phase (RP) mixed-mode cation-exchange (CX) column using acetonitrile (ACN) and water mobile phase with phosphoric acid buffer and UV detected at 200nm and 220nm.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN – 10% |
| Buffer | H3PO4 – 0.5% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200, 220 nm |
| Class of Compounds | Heterocycle, Amine, Hydrophilic, Ionizable |
| Analyzing Compounds | Dicyandiamide, 1,2,4-Triazole, 3-Methylpyrazole |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options3-Methylpyrazole
Dicyandiamide

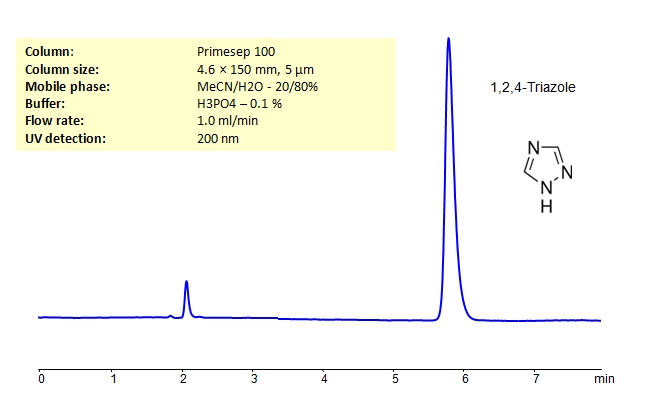
HPLC of Determination of 1,2,4-Triazole on Primesep 100 Column
December 17, 2019
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 20/80% |
| Buffer | H3PO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 nm |
| Class of Compounds |
Heterocyclic, Hydrophilic, Base |
| Analyzing Compounds | 1,2,3-Triazole |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options
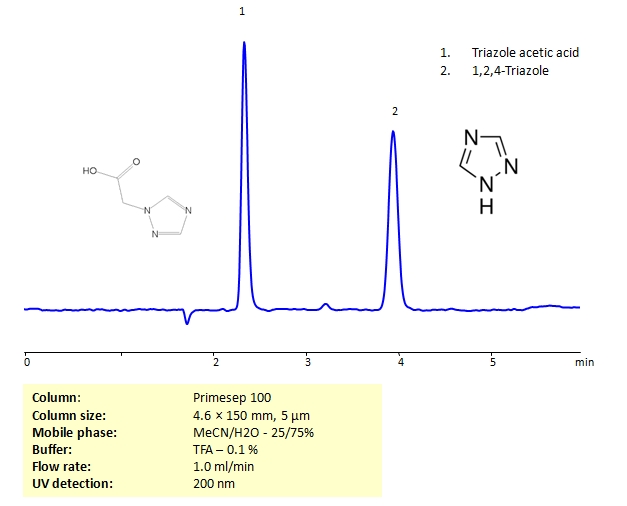
HPLC Separation of Triazole acetic acid and 1,2,4-Triazole on Primesep 100 Column
December 13, 2019
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 25/75% |
| Buffer | TFA – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | CAD (Corona) MS- compatible mobile phase |
| Class of Compounds |
Heterocyclic, Hydrophilic, Base |
| Analyzing Compounds | Triazole acetic acid, 1,2,3-Triazole |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsTriazole acetic acid