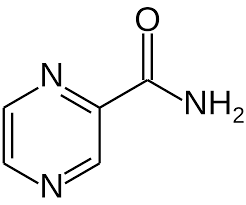
| CAS Number | 98-96-4 |
|---|---|
| Molecular Formula | C5H5N3O |
| Molecular Weight | 123.11 |
| InChI Key | IPEHBUMCGVEMRF-UHFFFAOYSA-N |
| LogP | -0.6 |
| Synonyms |
|
Applications:
HPLC Separation of Pyrazinecarboxamide and Related Compounds
June 25, 2020
![]()
View on hplc.cloud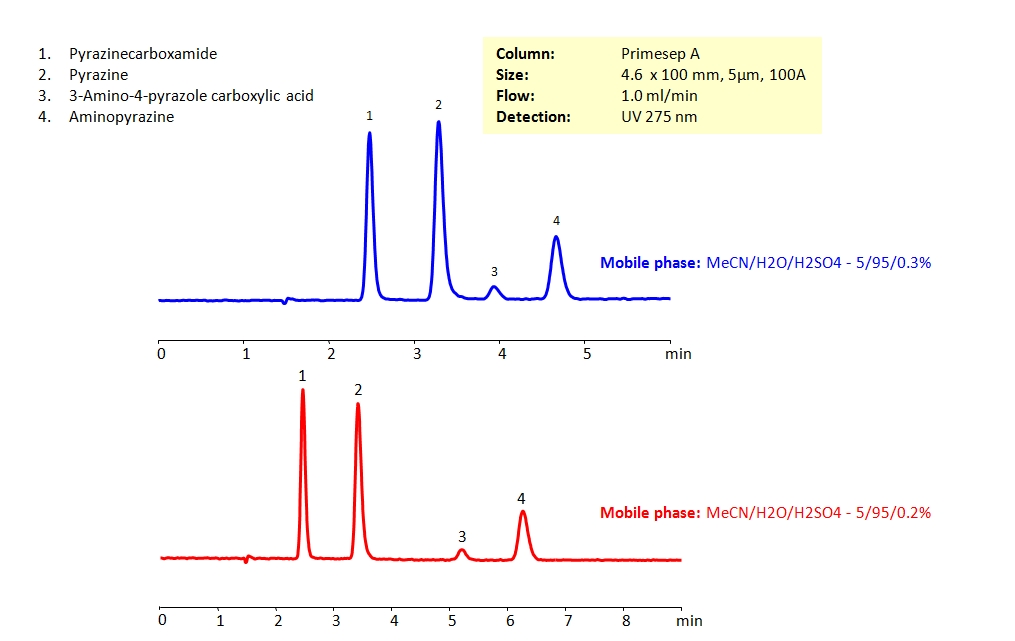
Pyrazinamide is a medication used in the treatment of infectious disease tuberculosis and is on the World Health Organization’s (WHO) list of essential medicines. It can be retained and separated from other pyrazine compounds, which are structurally similar and can be difficult to separate in reverse-phase HPLC, by using Primesep A mixed-mode column. Primesep A’s stationary phase is embedded with strong acidic ion-pairing groups. The analytical method is isocratic and uses the mobile phase of acetonitrile (ACN) and water with sulfuric acid (H2SO4) as buffer and UV detection at 275nm.
| Column | Primesep A, 4.6×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 5/95% |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV 275 nm |
| Class of Compounds | Hydrophilic, Heterocyclic, Aromatic |
| Analyzing Compounds | Pyrazine, Aminopyrazine, Pyrazinamide, 3-Aminopyrazole-4-carboxylic acid |
Application Column
Primesep A
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options3-Aminopyrazole-4-carboxylic acid
Pyrazinamide
Pyrazine