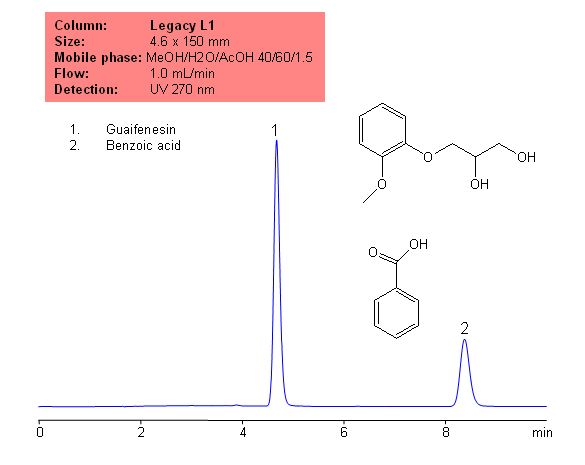
Application Notes: Guaifenesin is common, over the counter expectorant. Guaifenesin contain no less than 98 percent and not more than 102 percent of the labeled amount of guaifenesin calculated on a dried basis, according to the USP methods. the The USP HPLC method for the analysis of guaifenesin was developed on our Legacy L1 column according to the US Pharmacopeia methodology. L1 classification is assigned to reversed-phase HPLC column containing C18 ligand. Support for the material is spherical silica gel with particles size 3-10 um and pore size of 100-120A.
Application Columns: Legacy L1 C18 HPLC column
Application compounds: Guaifenesin, benzoic acid
Mobile phase: MeOH/H2O/AcOH 40:60:1.5
Detection technique: UV
Reference: USP 35- NF30
| Column | Legacy L1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeOH/H2O/AcOH 40/60/1.5 |
| Buffer | NaH2PO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Antibiotics, Hydrophobic, Ionizable, Acid |
| Analyzing Compounds | Guaifenesin, Benzoic acid |
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select optionsGuaifenesin





