| CAS Number | 303-38-8 |
|---|---|
| Molecular Formula | C7H6O4 |
| Molecular Weight | 154.121 |
| InChI Key | GLDQAMYCGOIJDV-UHFFFAOYSA-N |
| LogP | 1.20 |
| Synonyms |
|
Applications:
HPLC Separation of Dihydroxybenzoic Acids on Newcrom B Column
April 24, 2020
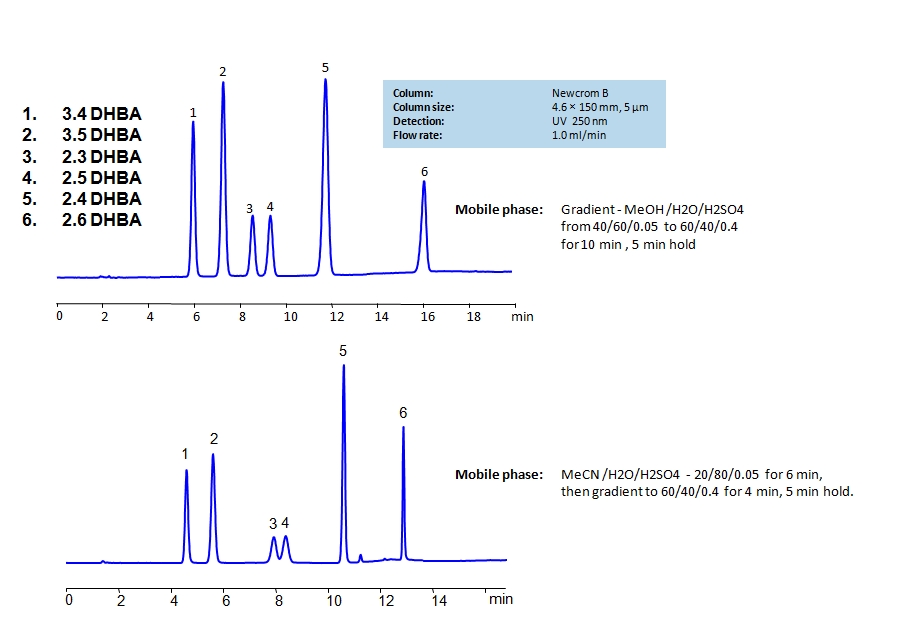
Dihydroxybenzoic acids are aromatic compounds consisting of a phenolic ring and a carboxylic acid. The six main compounds are structurally similar and are difficult to separate in reverse-phase HPLC. The can be separated by using a mixed-mode Newcrom B column with the mobile phase having either methanol (MeOH) or acetonitrile (ACN) as an organic modifier having different retention characteristics. Using the gradient of organic modifier, water and sulfuric acid (H2SO4) as buffer, dihydroxybenzoic acids can be separated and UV detected at 250nm.
| Column | Newcrom B, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | 3.4-dihydroxybenzoic acid, 3.5-dihydroxybenzoic acid,2,4-dihydroxybenzoic acid, 2,5-dihydroxybenzoic acid, 2,3-dihydroxybenzoic acid, 2,6-dihydroxybenzoic acid |
Application Column
Newcrom B
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select options2,4-Dihydroxybenzoic Acid
2,5-Dihydroxybenzoic Acid
2,6-Dihydroxybenzoic acid
3,4-Dihydroxybenzoic Acid
3,5-Dihydroxybenzoic Acid

Generic Screening Method for Complex Mixtures
October 15, 2015
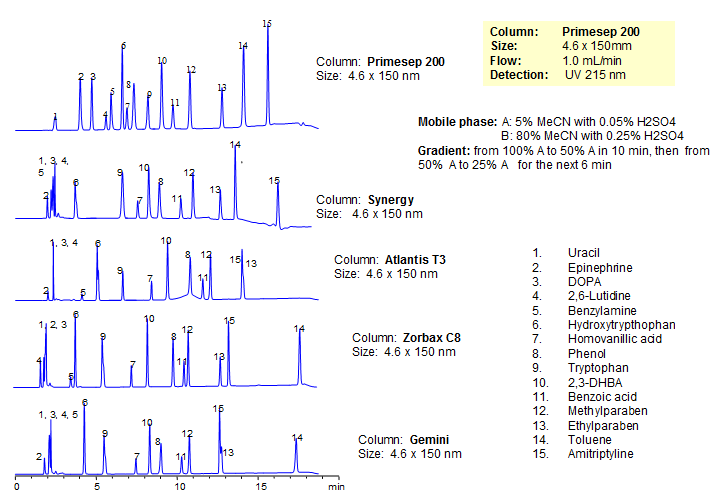
| Column | Primesep 200, 4.6*150 mm 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 215 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Uracil, Epinephrine, DOPA, 2,6-Lutidine, Benzylamine, Hydroxytrypthophan, Homovanillic acid, Phenol, Tryptophan , 2,3-DHBA, Benzoic acid, Methylparaben, Ethylparaben, Toluene, Amitriptyline |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options2,6-Lutidine
Amitriptyline
Benzoic Acid
Benzylamine
DOPA (3,4-dihydroxy-L-phenylalanine)
Epinephrine
Ethylparaben
Homovanillic Acid
Hydroxytryptophan
Methylparaben
Phenol
Toluene
Tryptophan
Uracil

HPLC Separation of Dihydroxybenzoic Acid
November 21, 2010
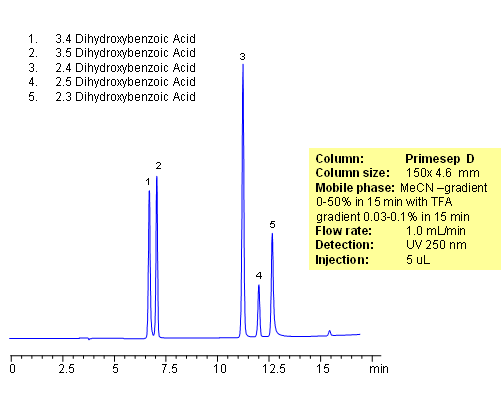
Separation of hydrophilic isomers is very difficult when only one mechanism of retention, like reversed-phase, is available. Isomers have very similar hydrophobic properties. In case of mixed-mode chromatography, small difference in hydrophobic and ionic properties allows to separate isomers. Isomers of dihydroxybenzoic acids are separated on a Primesep D column with good selectivity and peak shape. This method can be used for separation of other hydrophilic acidic compounds as well as hydrophobic basic compound.
| Column | Primesep D, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN |
| Buffer | TFA |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | 3.4-dihydroxybenzoic acid, 3.5-dihydroxybenzoic acid,2,4-dihydroxybenzoic acid, 2,5-dihydroxybenzoic acid, 2,3-dihydroxybenzoic acid |
Application Column
Primesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options2,4-Dihydroxybenzoic Acid
2,5-Dihydroxybenzoic Acid
3,4-Dihydroxybenzoic Acid
3,5-Dihydroxybenzoic Acid

Mixed-Mode Separation of Dihydroxybenzoic Acids
October 12, 2005
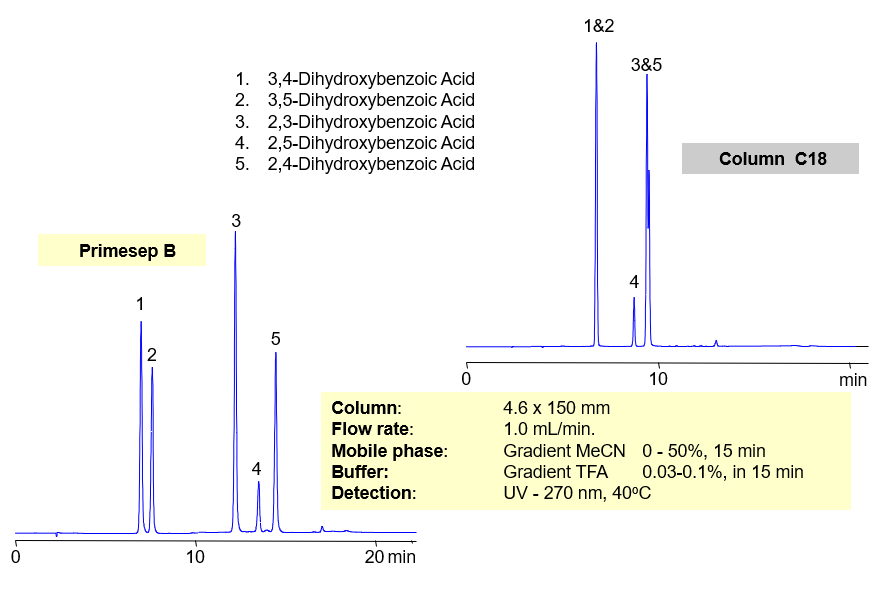
Primesep B offers better selectivity over traditional, reversed-phase C18 columns for separating regioisomers of aromatic dihydroxybenzoic acids (2,3-dihydroxybenzoic, 2,4-dihydroxybenzoic, 2,5-dihydroxybenzoic 3,4-dihydroxybenzoic, 3,5-dihydroxybenzoic acids) Primesep B combines reversed-phase and anion-exchange mechanism with a mass spec compatible mobile phase of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA).
| Column | Primesep B, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN |
| Buffer | TFA |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | 3.4-dihydroxybenzoic acid, 3.5-dihydroxybenzoic acid,2,4-dihydroxybenzoic acid, 2,5-dihydroxybenzoic acid, 2,3-dihydroxybenzoic acid |
Application Column
Primesep B
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options2,4-Dihydroxybenzoic Acid
2,5-Dihydroxybenzoic Acid
3,4-Dihydroxybenzoic Acid
3,5-Dihydroxybenzoic Acid
Carboxylic Acids