| CAS Number | 62624-30-0 |
|---|---|
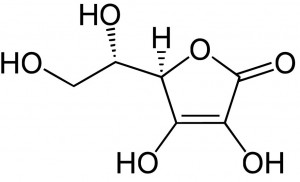
| Molecular Formula | C6H8O6 |
| Molecular Weight | 176.124 |
| InChI Key | CIWBSHSKHKDKBQ-UHFFFAOYSA-N |
| LogP | -2.24 |
| Synonyms |
|
Applications:
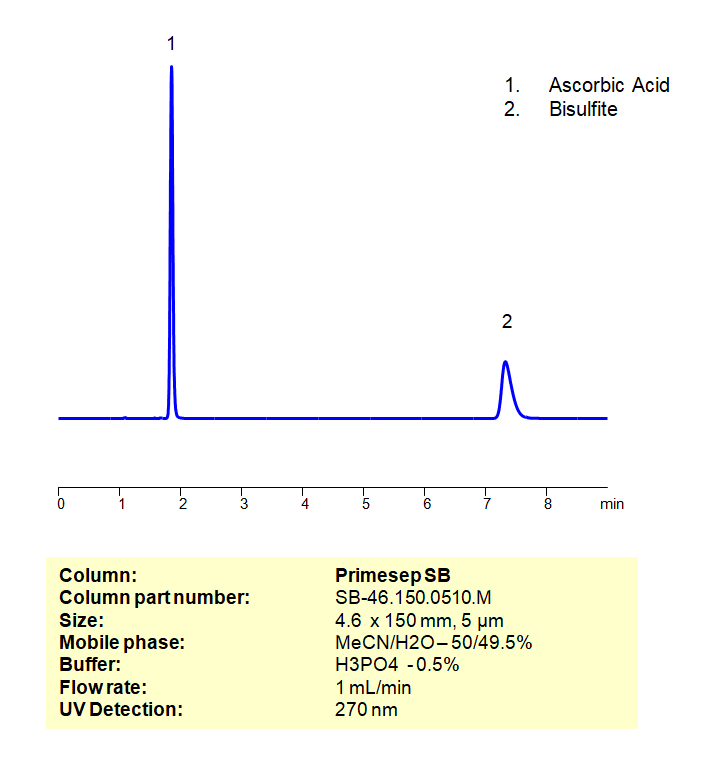
HPLC Isocratic Method for Analysis of Ascorbic Acid and Sodium Metabisulfite on Primesep SB Column
August 22, 2023
HPLC Method for Analysis of Ascorbic Acid, Sodium metabisulfite on Primesep SB by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Ascorbic Acid, Sodium metabisulfite
*The metabisulfite ion (S2O52−) is hydrolyzed to bisulfite (HSO3−) in water. Sodium metabisulfite is a white crystalline or powder solid. It has many uses, but some of it’s more prominent are: as the source of SO2 in wine, as a bleaching agent in the production of Coconut cream, and added to anesthetic solutions to prevent oxidation to improve the shelf life of the solution. Ascorbic is found naturally in citrus fruits and many vegetables. As a medication, it is used to prevent or treat low levels of vitamin C, since it is that vitamin. Vitamin C is needed to maintain the health of skin, cartilage, teeth, bone, and blood vessels. Ascorbic Acid and Sodium Metabisulfite can be separated, retained, and analyzed on a Primesep SB mixed-mode stationary phase column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a phosphoric acid (H3PO4) buffer. This analysis method can be detected in the UV 270 nm.
| Column | Primesep SB, 4.6 x 150 mm, 5 µm, 100 A |
| Mobile Phase | MeCN/H2O – 50/49.5% |
| Buffer | H3PO4 – 0.5% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds | Acid, Hydrophilic, Ionizable, Disinfectant, Antioxidant, Preservative agent. |
| Analyzing Compounds | Ascorbic Acid, Sodium metabisulfite |
Application Column
Primesep SB
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Sodium metabisulfite

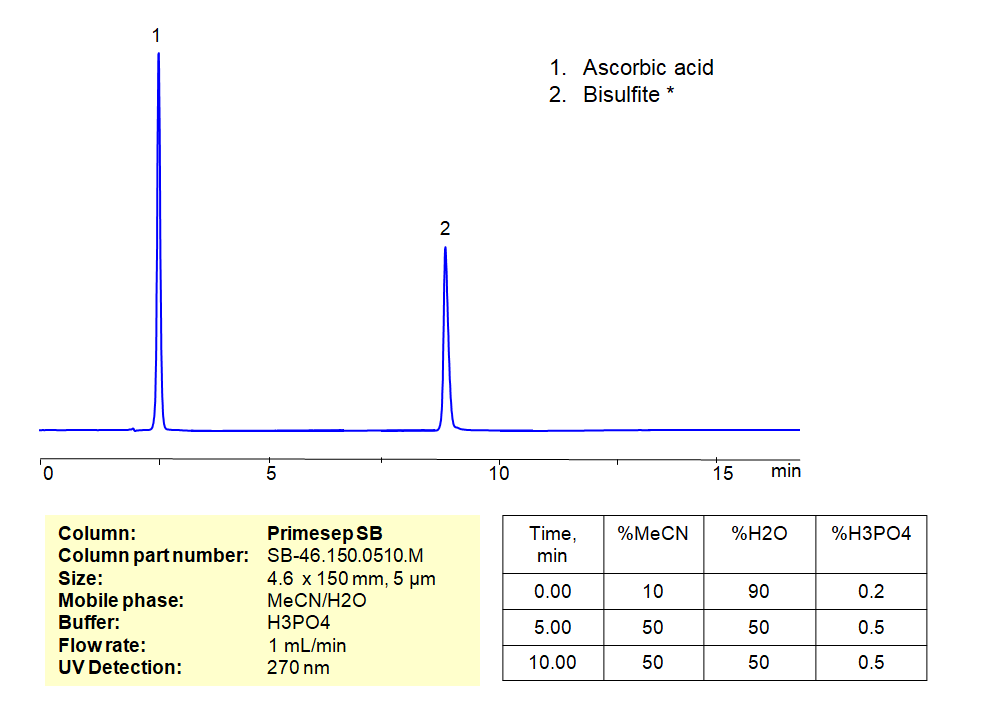
HPLC Method for Analysis of Ascorbic Acid and Sodium Metabisulfite on Primesep SB Column
August 22, 2023
HPLC Method for Analysis of Ascorbic Acid, Sodium metabisulfite on Primesep SB by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Ascorbic Acid, Sodium metabisulfite
*The metabisulfite ion (S2O52−) is hydrolyzed to bisulfite (HSO3−) in water. Sodium metabisulfite is a white crystalline or powder solid. It has many uses, but some of it’s more prominent are: as the source of SO2 in wine, as a bleaching agent in the production of Coconut cream, and added to anesthetic solutions to prevent oxidation to improve the shelf life of the solution. Ascorbic is found naturally in citrus fruits and many vegetables. As a medication, it is used to prevent or treat low levels of vitamin C, since it is that vitamin. Vitamin C is needed to maintain the health of skin, cartilage, teeth, bone, and blood vessels. Ascorbic Acid and Sodium Metabisulfite can be separated, retained, and analyzed on a Primesep SB mixed-mode stationary phase column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a phosphoric acid (H3PO $) buffer. This analysis method can be detected in the UV 270 nm.
| Column | Primesep SB, 4.6 x 150 mm, 5 µm, 100 A |
| Mobile Phase | MeCN/H2O |
| Buffer | H3PO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds | Acid, Hydrophilic, Ionizable, Disinfectant, Antioxidant, Preservative agent. |
| Analyzing Compounds | Ascorbic Acid, Sodium metabisulfite |
Application Column
Primesep SB
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Sodium metabisulfite

HPLC Method for Analysis of Maleic acid, Ascorbic acid, Nicotinic acid, Fumaric acid and Oxalic acid on BIST™ A+ Column
July 8, 2022
Separation type: Bridge Ion Separation Technology, or BIST™ by SIELC Technologies
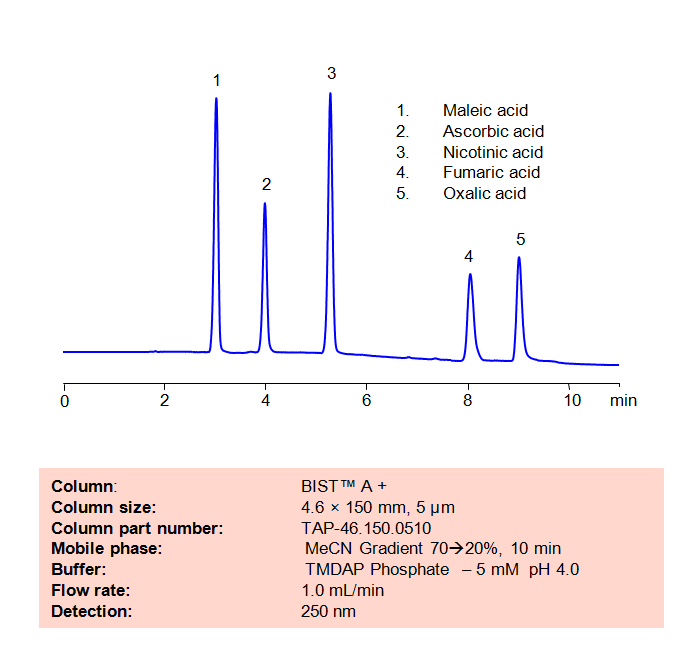
High Performance Liquid Chromatography (HPLC) Method for Analysis of Maleic acid, Ascorbic acid, Nicotinic acid, Fumaric acid and Oxalic acid
The maleate ion from Maleic acid is a popular ingredient as the maleate salt in several different drugs, including Methergine, Pyrilamine, and Carfenazine, among others. Nicotinic acid, also known as Niacin or Vitamin B3, is an essential nutrient for the human body and is sometimes taken as a treatment for high cholesterol. Aconitic acid is a key intermediary in the citric acid cycle, and is also used a flavoring agent and in the production of rubbers and plastics. Fumaric acid is a popular preservative and food additive with a fruit-like taste. Using SIELC’s newly introduced BIST™ method, a mixture of these organic acids can be separated on a negatively-charged, cation-exchange BIST™ A+ column, contrary to conventional chromatographic wisdom. There are two keys to this retention method: 1) a multi-charged, positive buffer, such as N,N,N’,N’-Tetramethyl-1,3-propanediamine (TMDAP), which acts as a bridge, linking the negatively-charged anion analytes to the negatively-charged column surface and 2) a mobile phase consisting mostly of organic solvent (such as MeCN) to minimize the formation of a solvation layer around the charged analytes. Other positively-charged buffers that can generate BIST™ include Calcium acetate and Magnesium acetate. Using this new and unique analysis method, these organic acids can be separated, retained, and detected through ELSD. This method is also compatible with Mass Spectrometry (LC-MS) and CAD.
Condition
| Column | BIST™ A+, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN Gradient |
| Buffer | TMDAP ( N,N,N’,N’-Tetramethyl-1,3-diaminopropane) phosphate – 5 mM pH 4.0 |
| Flow Rate | 1.0 ml/min |
| Detection | UV 250 nm |
Description
| Class of Compounds | Acids, Organic acid |
| Analyzing Compounds | Maleic acid, Ascorbic acid, Nicotinic acid, Fumaric acid and Oxalic acid |
Application Column
BIST A+
BIST™ columns offer a unique and effective way to achieve separations that were traditionally challenging or even impossible with other HPLC columns. With the use of a special mobile phase, these ion exchange columns provide very strong retention for analytes with the same charge polarity as the stationary phase, unlocking new chromatography applications. What makes BIST™ columns stand out is their proprietary surface chemistry, which results in superior selectivity, resolution, and sensitivity. These columns offer a simple, efficient solution for a variety of analytical challenges, making them an excellent choice for researchers and analysts across many different fields. To learn more about the technology that powers BIST™ columns and to explore related applications, check out https://BIST.LC.
Select optionsFumaric Acid
Maleic Acid
Nicotinic Acid/Niacin (3-pyridinecarboxylic acid)
Oxalic Acid

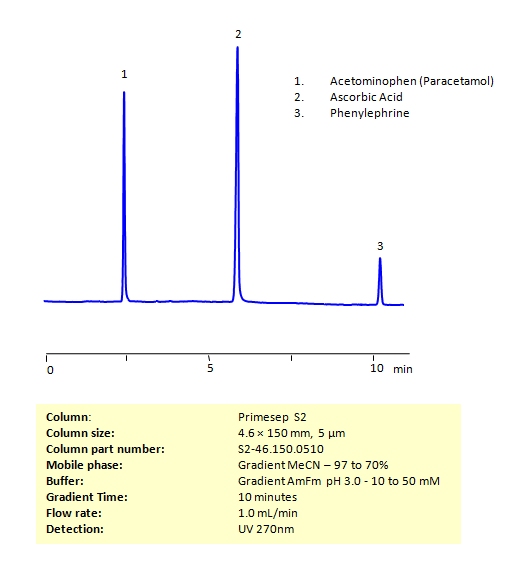
HPLC Method for the Determination of Acetaminophen, Phenylephrine, and Ascorbic Acid on Primesep S2 Column
October 5, 2021
Separation type: Liquid Chromatography Mixed-mode
High Performance Liquid Chromatography (HPLC) Method for Analysis of Acetaminophen, Phenylephrine, and Ascorbic Acid
Acetaminophen (or paracetamol) is one of the most popular over-the-counter painkillers all over the world (in the US it is best known under the brand name Tylenol). Ascorbic acid (also known as Vitamin C) helps your body build up muscle, cartilage, and other important building blocks in your body. Phenylephrine is a common over-the-counter decongestant, but can also be used for pupil dilation and hemorrhoid treatment. While all of these compounds are available at your local pharmacy, Vitamin C intake can reduce the body’s ability to break down acetaminophen.
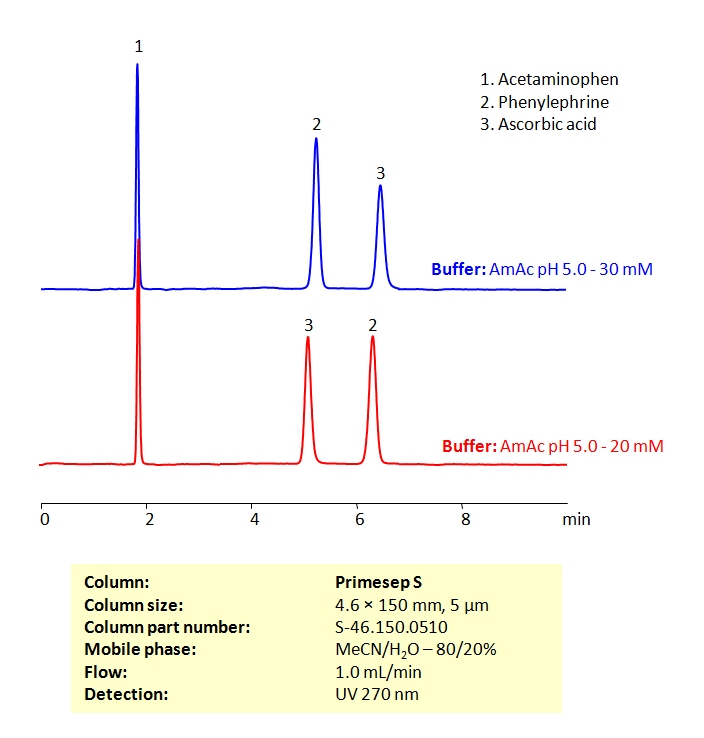
All three of these compounds can be measured at low UV. Using a Primsep S normal phase column and a mobile phase consisting of water and acetonitrile (MeCN) with an Ammonium acetate (AmAc) buffer, these three compounds can be separated and UV detected at 270 nm. Varying the buffer concentration changes the order in which phenylephrine and ascorbic acid are retained. This method is compatible with Mass Spectrometry.
| Column | Primesep S2, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 97 to 70% |
| Buffer | Gradient Ammonium Formate pH 3.0 – 10 to 50 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 270 nm MS- compatible mobile phase |
| Class of Compounds | Drug, Acid |
| Analyzing Compounds | Acetaminophen, Phenylephrine, and Ascorbic Acid |
Application Column
Primesep S2
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAscorbic Acid
Phenylephrine
Phenylephrine hydrochloride

HPLC Method for the Determination of Acetaminophen, Phenylephrine, and Ascorbic Acid on Primesep S Column
October 5, 2021
Separation type: Liquid Chromatography Mixed-mode
High Performance Liquid Chromatography (HPLC) Method for Analysis of Acetaminophen, Phenylephrine, and Ascorbic Acid
| Column | Primesep S, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 80/20% |
| Buffer | Ammonium acetate pH 5.0 |
| Flow Rate | 1.0 ml/min |
| Detection | UV 270nm MS- compatible mobile phase |
| Class of Compounds | Drug |
| Analyzing Compounds | Acetaminophen, Phenylephrine, and Ascorbic Acid |
Application Column
Primesep S
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAscorbic Acid
Phenylephrine
Phenylephrine hydrochloride

HPLC Determination of Ascorbic Acid in Strawberry Juice
May 6, 2019
FlipLC™ is an alternative method to avoid the interference of most of the contaminants by the use of an isolation column and a high pressure switching valve before the separation column. This method allows sample cleaning and analyte separation in one automated process. The isolation column and the separation column should have orthogonal retention characteristics to operate efficiently in this setup. Mixed-mode columns with reverse phase and ion-exchange characteristics were used in this analysis.
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep SB
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options
HPLC Method for Analysis of Ascorbic Acid and Sodium Metabisulfite on Newcrom BH Column
February 28, 2018
HPLC Method for Analysis of Ascorbic Acid, Sodium metabisulfite, Sodium bisulfite on Newcrom BH by SIELC Technologies
*The metabisulfite ion (S2O52−) is hydrolyzed to bisulfite (HSO3−) in water. Sodium metabisulfite is a white crystalline or powder solid. It has many uses, but some of it’s more prominent are: as the source of SO2 in wine, as a bleaching agent in the production of Coconut cream, and added to anesthetic solutions to prevent oxidation to improve the shelf life of the solution. Ascorbic is found naturally in citrus fruits and many vegetables. As a medication, it is used to prevent or treat low levels of vitamin C, since it is that vitamin. Vitamin C is needed to maintain the health of skin, cartilage, teeth, bone, and blood vessels. Ascorbic Acid and Sodium Metabisulfite can be separated, retained, and analyzed on a Newcrom BHmixed-mode stationary phase column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a phosphoric acid (H3PO $) buffer. This analysis method can be detected in the UV 275 nm.
| Column | (Column variation not found) |
| Mobile Phase | MeCN/H2O – 50/50% |
| Buffer | H3PO4 – 0.5% |
| Flow Rate | 0.5 ml/min |
| Detection | UV 275nm |
| Class of Compounds | Acid |
| Analyzing Compounds | Ascorbic Acid, Sodium metabisulfite, Sodium bisulfite |
High Performance Liquid Chromatography (HPLC) Method for Analysis of Ascorbic Acid, Sodium metabisulfite, Sodium bisulfite
Application Column
Newcrom BH
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsPrimesep SB
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsSodium bisulfite
Sodium metabisulfite

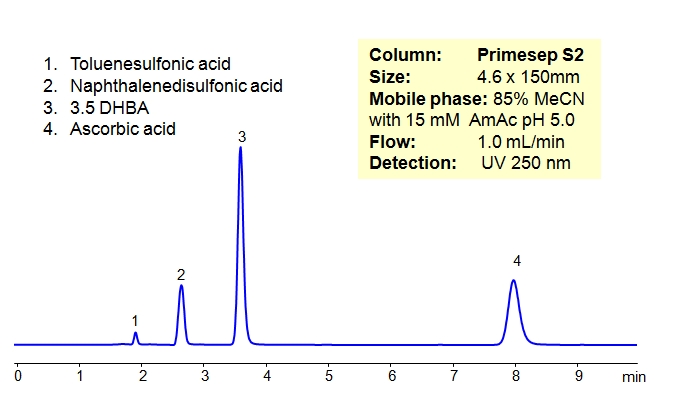
HPLC Separation of Organic Acids in HILIC and Anion-Exclusion Mode on Primesep S2 Column
July 14, 2011
Organic acids were separated on a HILIC/cation-exchange column in HILIC/anion-exclusion mode. This column can be used for analysis of polar compounds in HILIC mode. If compounds are ionizable, additional mode of interaction can be added (cation-exchange or anion-exclusion).
| Column | Primesep S2, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 85/15% |
| Buffer | AmAc pH 5.0 15 mM |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD, 50C UV 250 nm |
| Class of Compounds |
Nucleosides, Hydrophilic, Ionizable |
| Analyzing Compounds | Toluenesulfonic acid, Naphthalenedisulfonic acid, 3.5 DHBA, Ascorbic acid, |
Application Column
Primesep S2
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options3,5-Dihydroxybenzoic Acid
Ascorbic Acid
Organic Acids
p-Toluenesulfonic Acid (PTSA)

HPLC Separation of Ascorbic and Dehydroascorbic Acids
November 21, 2010
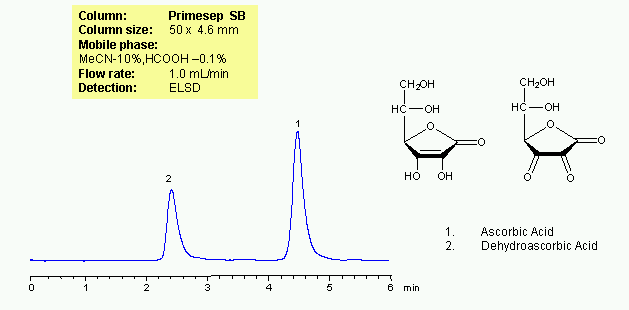
Ascorbic acid is a vital vitamin for humans. It also serves as an antioxidant. Dehydroascorbic acid is an oxidation product of ascorbic acid. Both molecules are very polar, and cannot be retained by reverse-phase mechanism. Generic method for HPLC separation of ascorbic and dehydroascorbic acid was developed on a Primesep SB mixed-mode HPLC column. Both compounds are well separated and produce excellent peak shape. Method can be used for analysis of both compounds in biofluids. Proteins from biofluids will not retain due to repulsion effect of the stationary phase. Compounds can be monitored by common detection techniques like UV, ELSD, CAD, and LC/MS.
| Column | Primesep SB , 4.6×50 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | Formic Acid |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Acid, Vitamin B₆, Hydrophobic, Ionizable |
| Analyzing Compounds | Ascorbic acid (Vitamin C), Dehydroascorbic acid |
Application Column
Primesep SB
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDehydroascorbic Acid

HPLC Analysis of Ascorbic Acid, Retinol (Vitamin A) and Vitamin E acetate in ACE Serum Active Ingredients
October 14, 2010
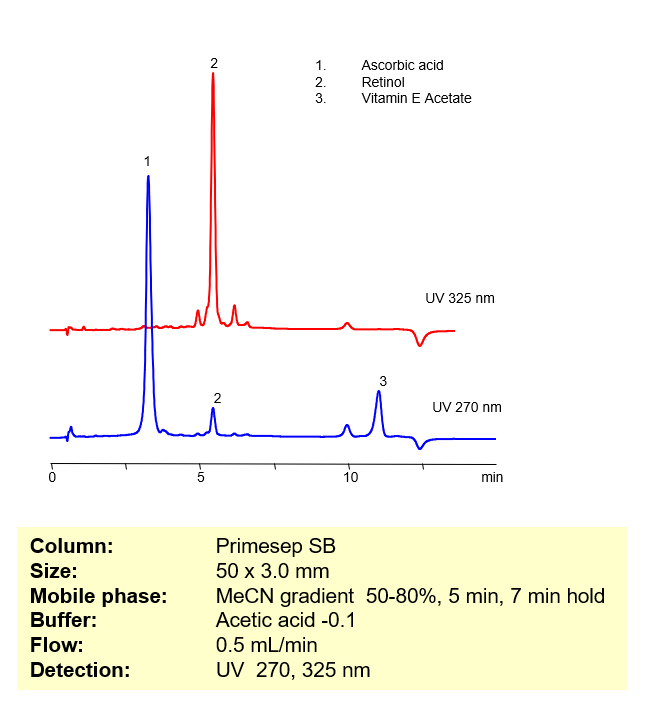
Mixed-mode HPLC columns allow for separation of compounds with drastically different properties. In this application, highly hydrophilic ascorbic acid is retained and separated from highly hydrophobic retinol (Vitamin A) and Vitamin E Acetate. Ascorbic acid is retained by anion-exchange mechanism and hydrophobic compounds are retained by reversed-phase mechanism. Compounds are monitored by UV detection. Mobile phase is compatible with LC/MS and method can be used to monitor basic and acidic compounds in bio-fluids (serum, blood, urine, saliva, etc.)
Retinol (Vitamin A) and Vitamin E Acetate
| Column | Primesep SB , 3.0×50 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | Acetic Acid |
| Flow Rate | 0.5 ml/min |
| Detection | UV 270, 325 |
| Class of Compounds |
Acid, Vitamin B₆, Hydrophobic, Ionizable |
| Analyzing Compounds | Ascorbic acid (Vitamin C), Retinol (Vitamin A) and Vitamin E Acetate |
Application Column
Primesep SB
Column Diameter: 3.2 mm
Column Length: 50 mm
Particle Size: 5 µm
Pore Size: 100 A
Retinol (Vitamin A)
Vitamin E Acetate

HPLC Analysis of Active Drug in a Formulation
October 4, 2010
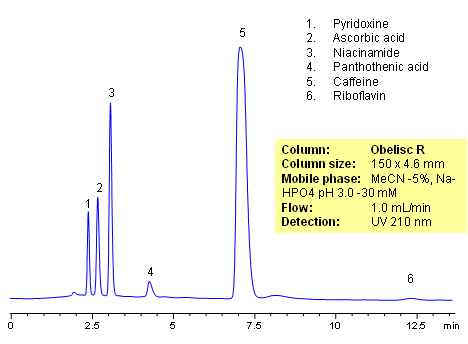
HPLC method for separation of active ingredients of drug/supplemental composition was developed on an Obelisc R trimodal HPLC column. Compounds are retained by combination of reversed-phase, cation-exchange and anion-exchange mechanisms. Compounds are well separated, and method can be used for quantitation of pyridoxine, ascorbic acid, niacinamide, pantothenic acid, caffeine and riboflavin in a mixture or as separate compounds in various complex mixtures. Various detection techniques can be applied for quantitation (ELSD, UV, LC/MS, Corona). This HPLC method can be adopted as general approach for analysis of active drug components in various formulations.
| Column | Obelisc R , 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O -5/95% |
| Buffer | NaHPO4 pH 3.0 – 30 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Vitamin B₆, Hydrophobic, Ionizable |
| Analyzing Compounds | Pyridoxine, Ascorbic acid, Niacinamide, Pantothenic acid, Caffeine, Riboflavin |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsAscorbic Acid
Caffeine
Niacinamide
Pantothenic Acid
Vitamin B6 (Pyridoxine)

HILIC Separation of Common Preservatives – Citric Acid, Ascorbic Acid and EDTA
July 16, 2009
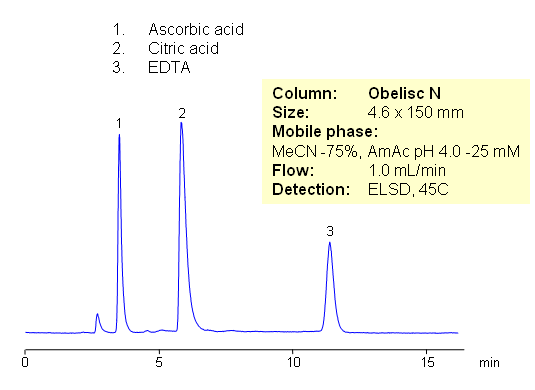
Citric acid, ascorbic acid, and EDTA are commonly used in food and pharmaceutical industry as preservatives. These compounds are very polar in nature. They are weak organic acids with limited UV activity. Retention and separation is achieved on HILIC mixed-mode Obelisc N column. All three compounds are retained by combination of strong HILIC and strong anion-exchange mechanisms. Separation can be monitored by ELSD, LC/MS, UV or Corona CAD. In contrast to other HILIC column, Obelisc N has two ionizable groups basic and acidic which provide ion-exchange interaction in addition to hydrophilic interaction. This allows to use less acetonitrile for HILIC separation.
| Column | Obelisc N , 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc pH 4.0 |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Acid, Vitamin B₆, Hydrophobic, Ionizable |
| Analyzing Compounds | Citric acid, ascorbic acid and EDTA |
Application Column
Obelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsEDTA (Ethylenediaminetetraacetic Acid)
Vitamin C

HPLC Separation of Vitamin C, Vitamin Group B, and Related Impurities
August 22, 2008
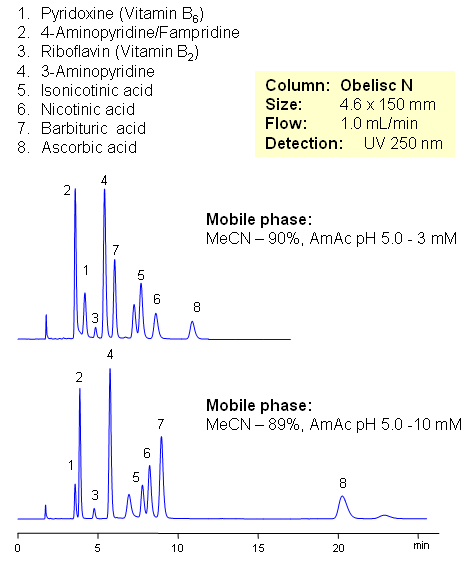
Vitamin C (ascorbic acid) and Vitamins Group B are separated on Obelisc N mixed-mode column. Method can be used in quantitation and determination of polar vitamins in various formulations and dietary supplements. HPLC method can be based on UV, Evaporative Light Scattering Detection (ESLD), RI or MS detection. Effect of sample matrix can be eliminated by changing mobile phase conditions. Buffer concentration, buffer pH and amount of ACN will affect every vitamin differently due to difference in polar and ionic properties.
| Column | Obelisc N , 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc pH 5.0 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Vitamin B₆, Hydrophobic, Ionizable |
| Analyzing Compounds | Pyridoxine, Ascorbic acid, Niacinamide, Pantothenic acid, Caffeine, Riboflavin, Barbituric Acid, 3- Aminopyrine |
Application Column
Obelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select options3-Aminopyridine
4-Aminopyridine/Fampridine
Ascorbic Acid
Barbituric Acid
Isonicotinic Acid
Nicotinic Acid/Niacin (3-pyridinecarboxylic acid)
Vitamin B2 (Riboflavin)
Vitamin B6 (Pyridoxine)

HPLC Separation of Organic Acids in HILIC Mode on Primesep N Column
December 6, 2007
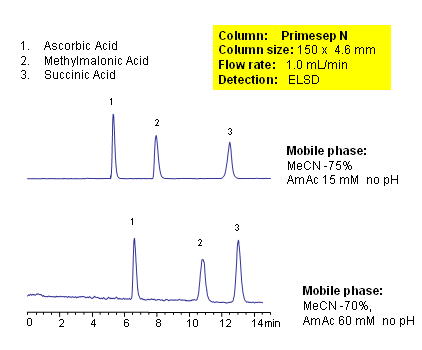
Ascorbic, methylmalonic and succinic are weak organic acids. Retention of these three acids is achieved on Primesep N column in HILIC mode using acetonitrile/water and ammonium acetate. Compounds are monitored by ELSD. Method can be used for determination of ascorbic acid (Vitamin C), methylmalonic acid and succinic acid in various matrices. Other polar organic acids can be analyzed on this HILIC column.
| Column | Primesep N , 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Acid, Vitamin B₆, Hydrophobic, Ionizable |
| Analyzing Compounds | Ascorbic acid (Vitamin C), Methylmalonic acid, Succinic acid |
Application Column
Primesep N
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsMethylmalonic Acid
Organic Acids
Succinic Acid

HPLC Separation of Two Vitamins: Different Polarity – Isocratic Methods
May 13, 2004
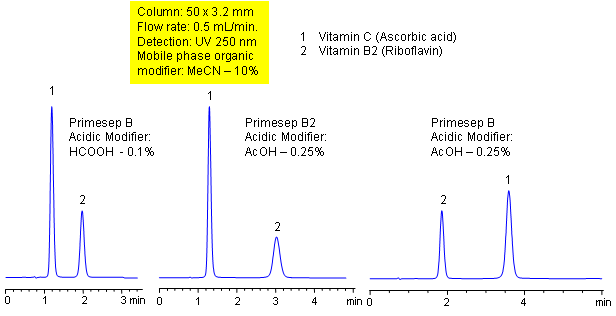
Primesep B and B2 columns separate Vitamins C (ascorbic acid) and B2 (riboflavin) with tunable selectivity. Peak order reversal is exhibited on these two columns with the same mobile phases due to their different polarity. The HPLC separation uses a mobile phase of water, acetonitrile (MeCN, ACN) and either formic or acetic acid (HOAc) with UV detection at 250 nm.
| Column | Primesep B, Primesep B2 , 3.2×50 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | Formic Acid, Acetic Acid |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Vitamin B₆, Hydrophobic, Ionizable |
| Analyzing Compounds | Vitamins C (ascorbic acid) and B2 (riboflavin) |
Application Column
Primesep B
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep B2
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAscorbic Acid
Vitamin B2 (Riboflavin)
Vitamin C