| CAS Number | 7440-50-8 |
|---|---|
| Molecular Formula | Cu |
| Molecular Weight | 63.546 |
| InChI Key | RYGMFSIKBFXOCR-UHFFFAOYSA-N |
| LogP | -0.570 |
| Synonyms |
|
Applications:
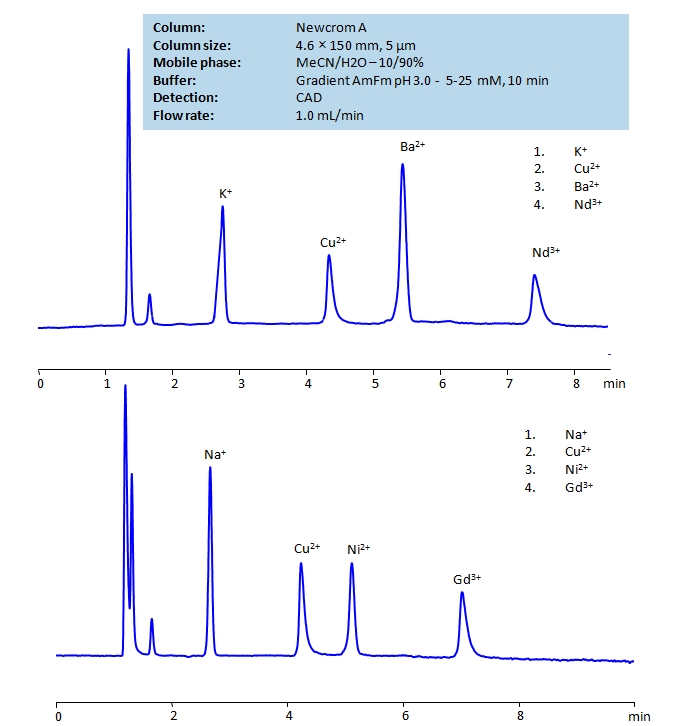
HPLC Determination of Ions on Newcrom A Column
March 24, 2020
| Column | Newcrom A, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN – 10% |
| Buffer | Gradient Ammonium formate pH 3.0 |
| Flow Rate | 1.0 ml/min |
| Detection | CAD |
| Class of Compounds | Hydrophilic, Metal, Ion |
| Analyzing Compounds | Sodium, Potassium, Copper, Nickel, Barium, Neodymium, Gadolinium |
Application Column
Newcrom A
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsCopper
Copper Sulfate
Gadolinium acetate
Neodymium
Nickel
Sodium

HPLC UV Analysis of Copper Ions in Salt Mixture
July 3, 2013
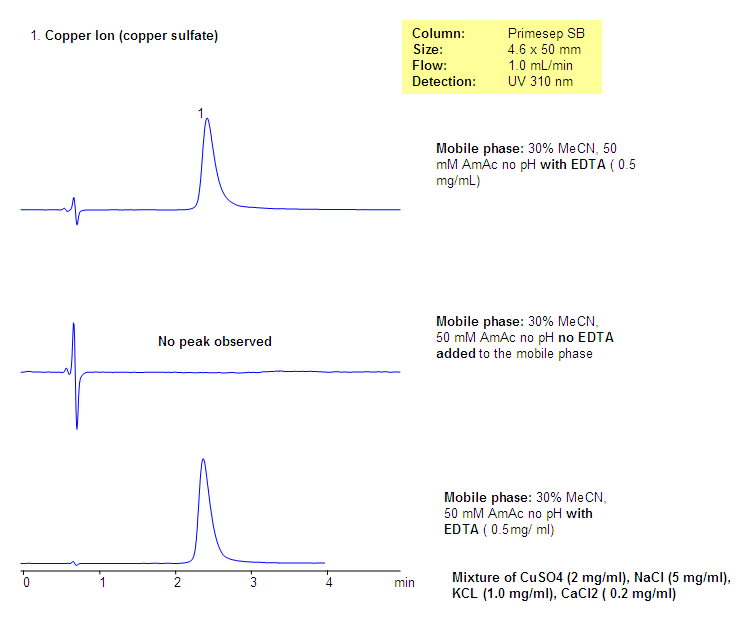
A unique approach for analysis of copper ions in a high salt content matrix was developed using EDTA as a visualization agent. Since copper ions can form colored complexes with EDTA, this property can be used for selective analysis of copper in the mixture with other cations, which do not form a colored complex with EDTA. Copper ion can be monitored by UV while other metals pass through the column undetected by UV. This method can be used to analyze copper ion at parts per million concentrations.
| Column | Primesep SB, 4.6×50 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | AmAc, EDTA |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 310 nm |
| Class of Compounds |
Ions |
| Analyzing Compounds | Copper |
Application Column
Primesep SB
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsCopper Sulfate

HPLC Retention of Copper Ion on Primesep 100 Mixed-Mode Cation-Exchange Columns
July 16, 2009
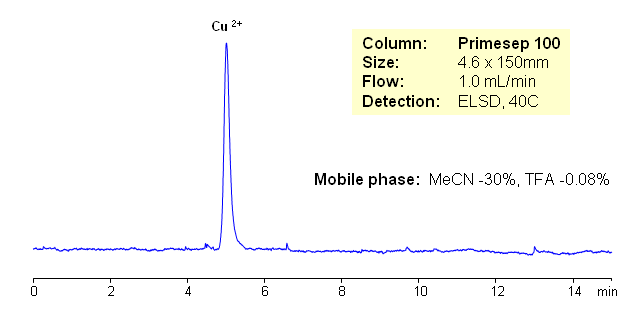
Copper ions are usually analyzed by ion-exchange chromatography with ion suppression detection. In this application copper ion is analyzed on mixed-mode cation-exchange column with ELSD detection. Copper is retained by cation-exchange mechanism. Other organic and inorganic cations can be analyzed by this approach. Retention time is controlled by amount of buffer or acid and by pH of the mobile phase.
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options