| CAS Number | 65-46-3 |
|---|---|
| Molecular Formula | C9H13N3O5 |
| Molecular Weight | 243.219 |
| InChI Key | UHDGCWIWMRVCDJ-XVFCMESISA-N |
| LogP | -2.33 |
| Synonyms |
|
Applications:
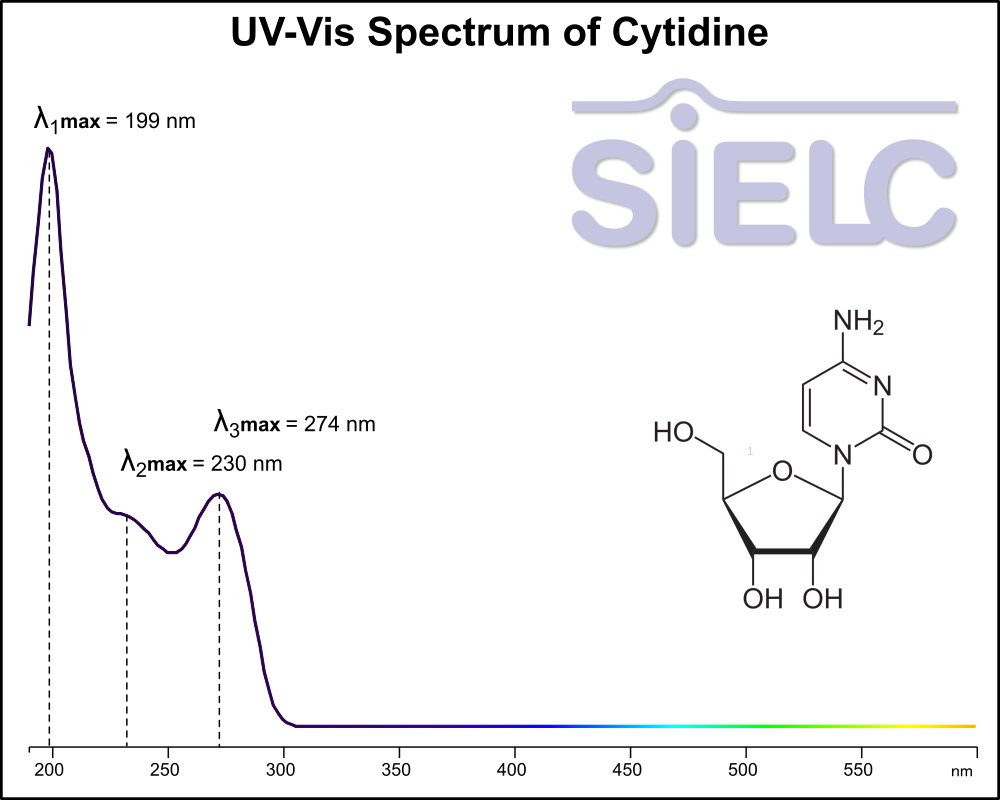
Uv-Vis Spectrum of Cytidine
February 2, 2026
If you are looking for optimized HPLC method to analyze Cytidine check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

HPLC Method for Separation of Cytosine, Deoxycytidine and Cytidine on BIST B+ Column
November 28, 2022
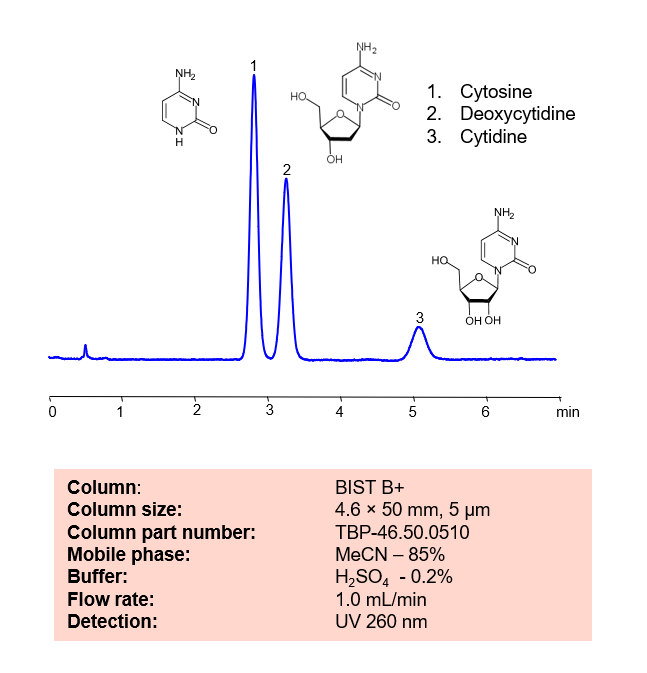
HPLC Method for Separation of Cytosine, Deoxycytidine, Cytidine on BIST B+ by SIELC Technologies.
Cytosine, also noted as C and Cyt, has the chemical formula C4H5N3O. In DNA, it pairs with Guanine to create a base pair. In RNA, it is synonymous with Uracil, being an interchangeable third base. Not only that, due to it’s instability, it can change into Uracil through spontaneous deamination.
Deoxycytidine is a deoxyribonucleoside with the chemical formula C9H13N3O4. It is a precursor for 5-aza-2′-cytidine, which is a treatment for myelodysplastic syndrome. It works through interfering with the methylation of the P15/INK4B gene. It can also be used as a biomarker for tumor diagnosis.
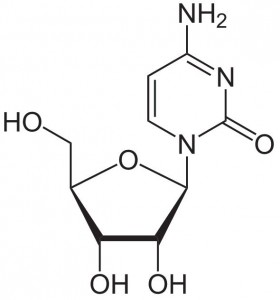
Cytidine, also noted as C or Cyd, is a nucleoside molecule with the chemical formula C9H13N3O5. It is primarily found in foods with high RNA contents, such as organ meets, brewer’s yeast, and beer. During digestion, Cyd is broken down into ribosyl pyrimidines.
Cytosine, Deoxycytidine, Cytidine can be retained and analyzed using the BIST B+ stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a sulfuric acid buffer. Detection is performed using UV.
Condition
| Column | BIST B+, 4.6 x 50 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 85% |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260 nm |
| Peak Retention Time | 2.8, 3.2, 5.1 min |
Description
| Class of Compounds | Nucleosides |
| Analyzing Compounds | Cytosine, Deoxycytidine, Cytidine |
Application Column
BIST B+
Column Diameter: 4.6 mm
Column Length: 50 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Cytosine
Deoxycytidine

HPLC Separation of Nucleosides and Deoxynucleosides
July 23, 2012
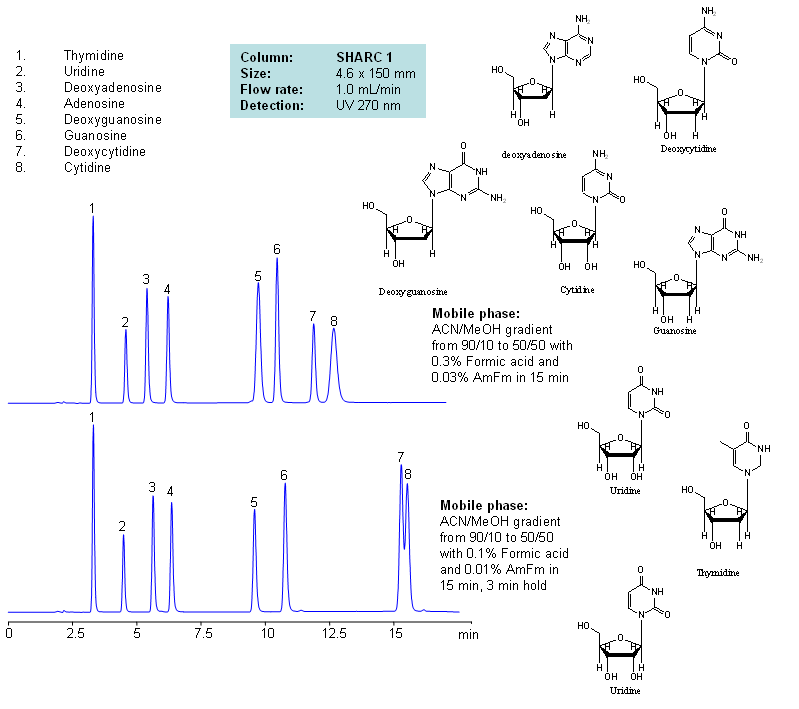
| Column | Sharc 1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH |
| Buffer | AmFm, Formic acid |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Thymidine, Uridine, Deoxyadenosine, Adenosine, Deoxyguanosine, Guanosine, Deoxycytidine, Cytidine |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select optionsCytidine
Deoxyadenosine
Deoxycytidine
Deoxyguanosine
Guanosine
Thymidine
Uridine

HPLC Separation of Cytidine and Cytosine Using the Hydrogen Bonding Method
June 18, 2012
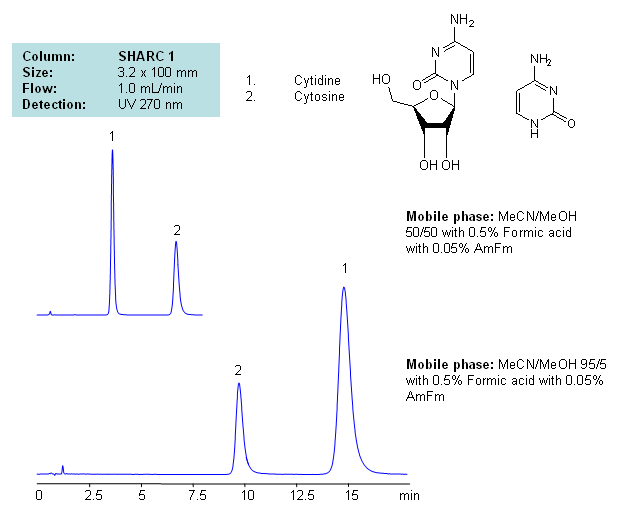
Application Notes: Nucleosides glycosylamines consisting of nucleobase linked to ribose or deoxyribose sugar. Nucleoside are building blocks for DNA and RNA. These compounds are very polar in nature and contain groups available for hydrogen bonding interaction. A method for separation of cytosine and cytidine was developed based on the strong dependence of retention time to the mobile phase composition. The mobile phase consists of acetonitrile and methanol. Order of elution for compounds depends on the amount of acetonitrile and methanol. Our method is compatible with LC/MS and preparative chromatography, and can be used for separation of other nucleobases and nucleotides.
Application Columns: SHARC 1, 3.2×100 mm, 5 um, 100A. To learn more about SHARC 1 columns click here. To order this column click here. To see more chromatographic separations check our web site.
Application Compounds: Cytosine and Cytidine
Detection Technique: UV, LC/MS
| Column | Sharc 1, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH |
| Buffer | AmFm, Formic acid |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Cytidine, Cytosine |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select optionsCytosine

HPLC Separation of Thymidine, Uridine, Adenosine, Guanosine, and Cytidine Using the Hydrogen Bonding Method
June 15, 2012
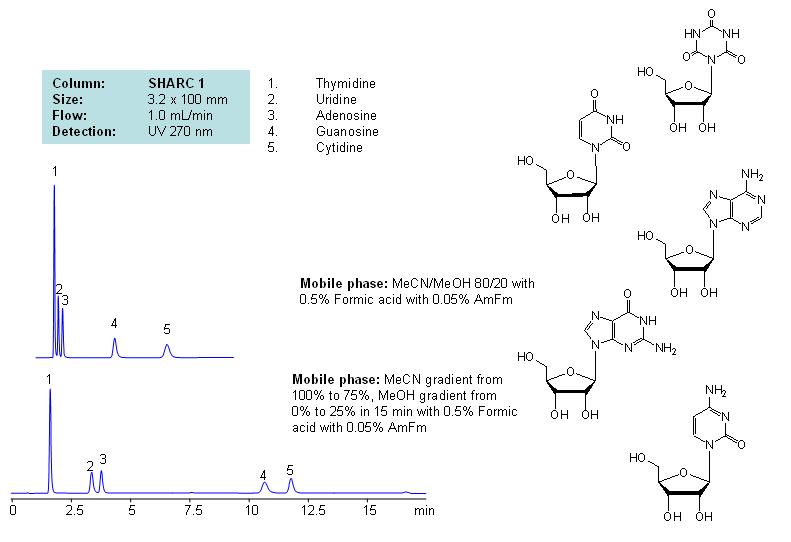
Application Notes: Nucleosides are glycosylamines consisting of nucleobase linked to ribose or deoxyribose sugar and are building blocks for DNA and RNA. These compounds are very polar and contain groups available for hydrogen bonding interaction. Thymidine, uridine, adenosine, guanosine and cytidine were separated using a hydrogen-bonding method. There is a strong correlation between the retention time and mobile phase composition. The strength of hydrogen-bonding interaction increases as the number of hydroxyls in the analytes increase. Additionally the order of elution for compounds depends on the ratio of the mobile phases: acetonitrile and methanol. Our method is compatible with LC/MS and preparative chromatography.
Application Columns: SHARC 1, 3.2×100 mm, 5 um, 100A, To learn more about SHARC 1 columns click here. To order this column click here. To see more chromatographic separations check our web site.
Application Compounds: Thymidine, uridine, adenosine, guanosine and cytidine
| Column | Sharc 1, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH |
| Buffer | AmFm, Formic acid |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Thymidine, Uridine, Adenosine, Guanosine, Cytidine |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select optionsCytidine
Guanosine
Thymidine
Uridine

HPLC Separation of Nucleic Bases at pH 4 and 5 on Obelisc N
March 3, 2007
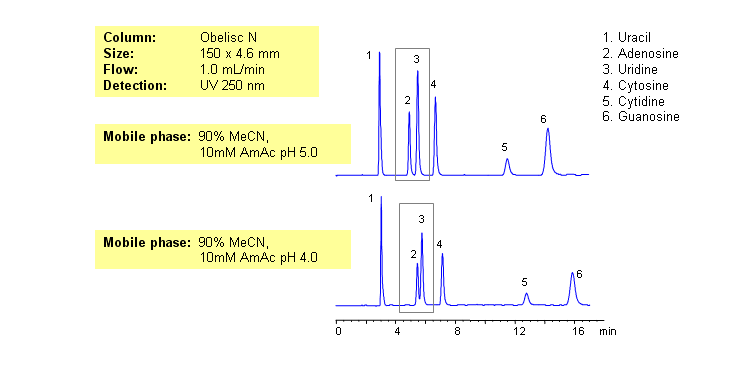
Nucleic bases are biological compounds found in genetic molecules (DNA, RNA). They can be separated on an Obelisc N column, which offers very polar characteristics and can be used with positively or negatively charged groups. Closely-eluted adenosine and uridine can be further separated by simply adjusting the pH of the mobile phase. Mobile phase is water and acetonitrile (MeCN, ACN) with Ammonium Acetate as buffer. UV detection at 250nm.
| Column | Obelisc N, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN -90% |
| Buffer | AmAc |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Uracil, Uridine, Adenosine, Guanosine, Cytidine, Cytosine |
Application Column
Obelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsCytidine
Cytosine
Guanosine
Uracil
Uridine