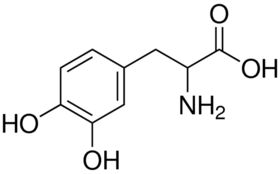
| CAS Number | 59-92-7 |
|---|---|
| Molecular Formula | C9H11NO4 |
| Molecular Weight | 197.190 |
| InChI Key | WTDRDQBEARUVNC-LURJTMIESA-N |
| LogP | -2.39 |
| Synonyms |
|
Applications:
Generic Screening Method for Complex Mixtures
October 15, 2015
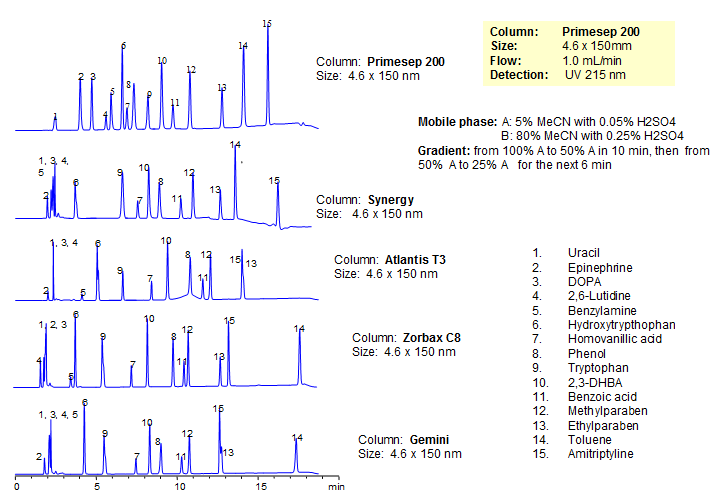
| Column | Primesep 200, 4.6*150 mm 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 215 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Uracil, Epinephrine, DOPA, 2,6-Lutidine, Benzylamine, Hydroxytrypthophan, Homovanillic acid, Phenol, Tryptophan , 2,3-DHBA, Benzoic acid, Methylparaben, Ethylparaben, Toluene, Amitriptyline |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options2,6-Lutidine
Amitriptyline
Benzoic Acid
Benzylamine
DOPA (3,4-dihydroxy-L-phenylalanine)
Epinephrine
Ethylparaben
Homovanillic Acid
Hydroxytryptophan
Methylparaben
Phenol
Toluene
Tryptophan
Uracil

HPLC Separation of Neurotransmitters
November 25, 2011
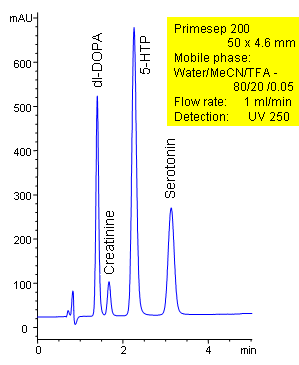
The neurotransmitters dl-DOPA, creatinine, hydroxytryptophan (5-HTP), and serotonin are separated in less than 4 minutes on a short 50 mm Primesep 200 column. The HPLC separation uses a mobile phase of water, acetonitrile (MeCN, ACN) and trifluoroacetic acid (TFA) and UV detection at 250 nm. Ion-pair reagents were not needed for retention of these polar, hydrophilic compounds, instead, a combination of ion-exchange and reversed-phase interactions were used.
| Column | Primesep 200, 4.6×50 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 80/20% |
| Buffer | TFA – 0.05% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | dl-DOPA, Creatinine, Hydroxytryptophan (5-HTP), Serotonin |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDOPA (3,4-dihydroxy-L-phenylalanine)
Hydroxytryptophan
Serotonin

HPLC Separation of Amino Acids in HILIC and Cation-Exchange Modes
July 14, 2011
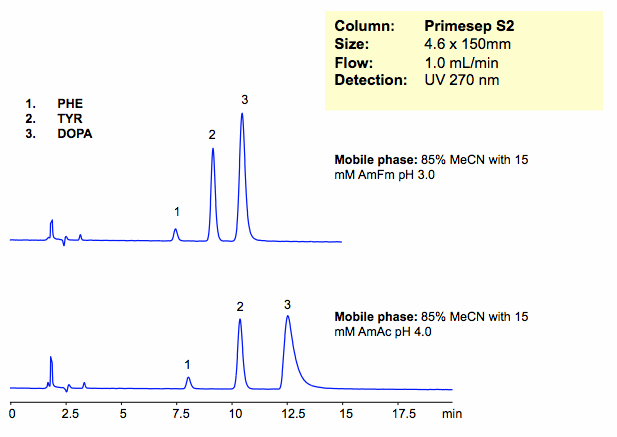
Amino acids can be retained and separated on a Primesep S2 column in HILIC/cation-exchange mode.
| Column | Primesep S2, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Monocarboxylic acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Dopa, Tyrosine, Phenylalanine |
Application Column
Primesep S2
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPhenylalanine
Tyrosine

HPLC Separation of Catecholamines on Primesep 100 Column with Phosphate Buffers
July 8, 2011
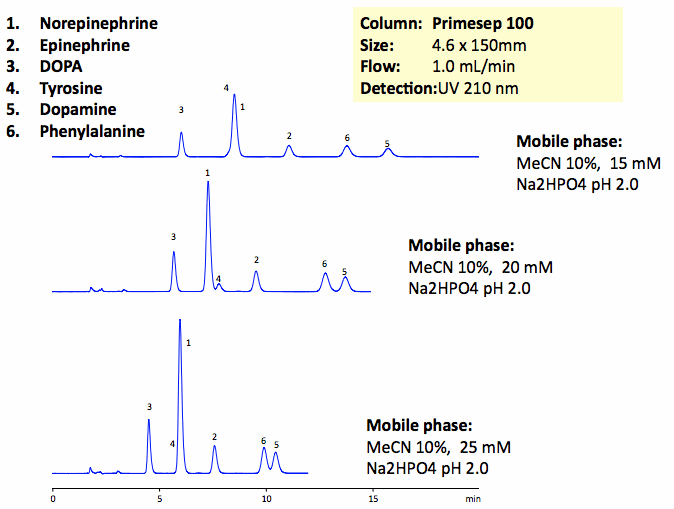
The catecholamine neurotransmitters are amino-acid derivatives of tyrosine. DOPA, tyrosine, phenylalanine, norepinephrine, epinephrine, and dopamine and baseline are resolved on a Primesep 100 column with UV-transparent phosphate buffer. This method can be used for analysis of catecholamines and related impurities in various matrices. Peak order and retention time can be changed by changing the amount of ACN, buffer concentration and buffer pH. Various buffers can be used to accommodate desired detection technique. Primesep 100 is a reversed-phase cation-exchange mixed-mode column that can be used for analysis of polar neutral, polar ionizable, polar zwitter-ionic, hydrophobic neutral, and hydrophobic ionic compounds in the same run. Column can be operated in reverse-phase, cation-exchange, anion-exclusion, HILIC and mixed-modes depending on the mobile phase selection and nature of analytes. Column is compatible with LC/MS and does not require use of ion-pairing reagents.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | Na2HPO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Tyrosine, DOPA, Phenylalanine, Norepinephrine, Epinephrine, Dopamine |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDopamine
Epinephrine
Norepinephrine
Phenylalanine
Tyrosine

Separation of Serotonin, Dopamine, and Related Compounds
August 22, 2008
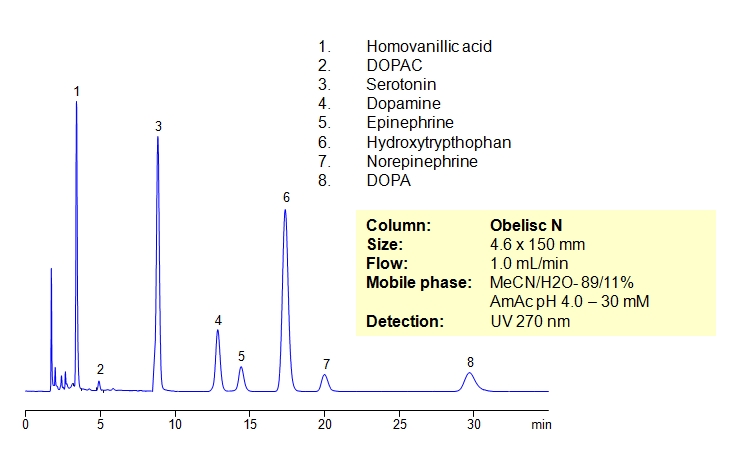
Catecholamines are chemical compounds derived from the amino acid tyrosine containing catechol and amine groups. Some of them are biogenic amines. Retention of compounds of the catecholamine pathway is achieved on Obelisc N column. All polar compounds are well retained by combination of HILIC and ion-exchange mechanisms. Obelisc N columns produce very good peak shapes for all analytes. The method is very sensitive to amount of ACN, buffer and buffer pH. The retention time changes with variation of the main parameters. This method can be used for quantitation of biogenic amines and related compounds (homovanillic acid, dihydroxyphenyl acetic acid, serotonin, dopamine, epinephrine, hydroxytryptophan, epinephrine and DOPA) in urine, blood and other biological fluids. Further optimization of this HPLC method can be used during screening and validation. Amines and acids can be analyzed in the same run and retained by a combination of polar organic mode, cation-exchange and anion-exchange modes. Various buffers within specified pH can be employed (ammonium formate, ammonium acetate, sodium phosphate, etc.).
| Column | Obelisc N, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc pH 4.0- 30 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Monocarboxylic acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Homovanillic acid, Dihydroxyphenyl acetic acid, Serotonin, Dopamine, Epinephrine, Hydroxytryptophan, DOPA |
Application Column
Obelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsDOPAC (Dihydroxyphenylacetic Acid)
Dopamine
Epinephrine
Homovanillic Acid
Hydroxytryptophan
Norepinephrine
Serotonin

HPLC Separation of Polar and Hydrophobic Drugs on Obelisc R and N
March 3, 2007
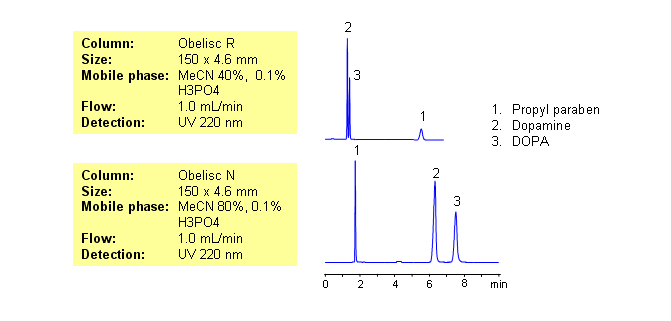
| Column | Obelisc N, Obelisc R 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H3PO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 220 nm |
| Class of Compounds |
Drug, Acid, Preservative, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Propyl paraben, Dopamine, DOPA |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsObelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsDopamine
Propylparaben

HPLC Separation of Compounds of Catecholamine Pathway
March 3, 2006
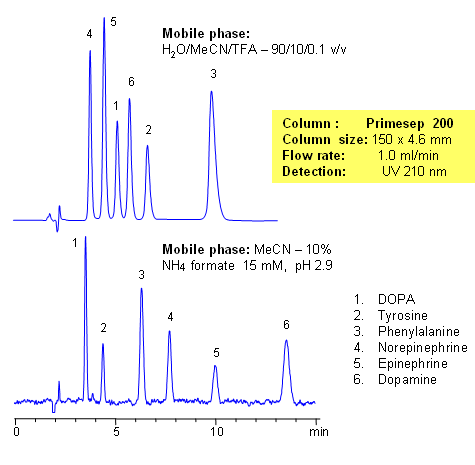
The catecholamine neurotransmitters are amino-acid derivatives of tyrosine. DOPA, tyrosine, phenylalanine, norepinephrine, epinephrine, and dopamine and baseline are resolved on a
Primesep 200 column with UV-transparent phosphate buffer. This method can be used for the analysis of catecholamines and related impurities in various matrices. The peak order and retention time can be changed by changing the amount of ACN, buffer concentration and buffer pH. Various buffers can be used to accommodate the desired detection technique. Primesep 200 is a reversed-phase cation-exchange mixed-mode column that can be used for analysis of polar neutral, polar ionizable, polar zwitterionic, hydrophobic neutral, and hydrophobic ionic compounds in the same run. Column can be operated in reverse-phase, cation-exchange, anion-exclusion, HILIC and mixed-modes depending on the mobile phase selection and the nature of the analytes. The column is compatible with LC/MS and does not require the use of ion-pairing reagents.
| Column | Primesep 200, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | TFA, AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Tyrosine, DOPA, Phenylalanine, Norepinephrine, Epinephrine, Dopamine |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDopamine
Epinephrine
Phenylalanine
Tyrosine
UV Detection

Bufferless Ion Separation (BLIS™) Chromatography of Amino Acids (1)
January 13, 2005
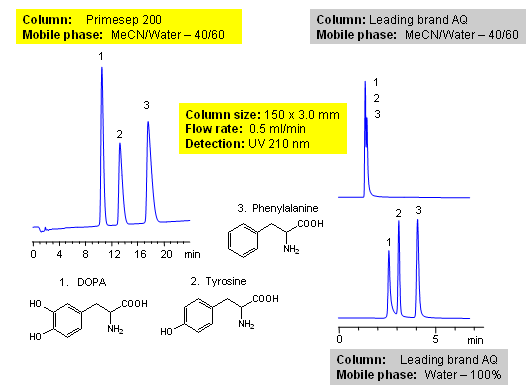
Amino acids are the building blocks of proteins and are widely used as supplements. Amino acids can exist in acidic, basic or zwitterionic form depending on the pH of their environment (mobile phase in this case). Primesep 200 is a reverse-phase column with embedded weak acidic ion-pairing groups which allows for retention and separation of amino acids without requiring a buffer making it ideal for use with UV detection, mass spectrometry (MS), evaporative light scattering detection (ELSD).
| Column | Primesep 200, 3.0×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 40/60% |
| Buffer | No |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Dopa, Phenylalanine, Tyrosine |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPhenylalanine
Tyrosine

HPLC Analysis of the Catecholamine Pathway
January 17, 2004
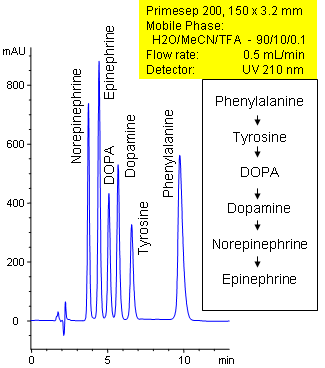
Primesep 200 separates catecholamines in the catecholamine pathway in 10 minutes. Phenylalanine, tyrosine, DOPA, dopamine, norepinephrine, and epinephrine are baseline resolved by a combination of reversed-phase, ion-exchange, and ion-exclusion mechanisms. Excellent peak shape results with a mass spec compatible mobile phase of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) with UV detection at 210 nm.
| Column | Primesep 200, 3.2×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 10/90% |
| Buffer | TFA – 0.1% |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Tyrosine, DOPA, Phenylalanine, Norepinephrine, Epinephrine, Dopamine |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDopamine
Epinephrine
Phenylalanine
Tyrosine