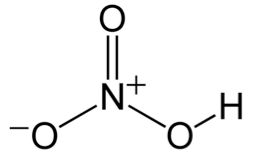
| CAS Number | 7697-37-2 |
|---|---|
| Molecular Formula | HNO3 |
| Molecular Weight | 63.012 |
| InChI Key | GRYLNZFGIOXLOG-UHFFFAOYSA-N |
| LogP | 0.21 |
| Synonyms |
|
Applications:
HPLC Separation of Inorganic Anions
November 21, 2010
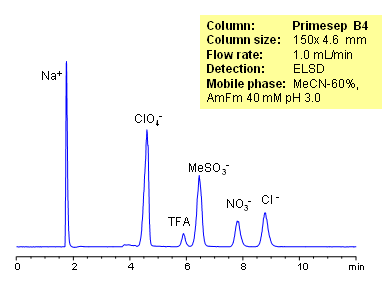
Organic and inorganic acids and ions can be separated on a Primesep B4 column based on their ionic properties. Method can be used for quantitation of residual acids in various products and sample matrices. Trifluoracetic, hydrochloric, methanesulfonic, and nitric acids are separated using ACN-water-ammonium formate. Ions can be detected by ELSD, CAD or LC/MS.
| Column | Primesep B4, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Ions, Hydrophilic, Ionizable |
| Analyzing Compounds | Sodium, Phosphate, Chloride, Nitrate, Sulfate, Iodide, Perchlorate, Trifluoracetic |
Application Column
Primesep B4
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsMethanesulfonic Acid
Nitrate
Nitric Acid
Organic Acids
Perchloric Acid
TFA (Trifluoroacetic Acid)

HPLC Separation of Potassium, Perchlorate, Methanesulfonic, Chloride, Bromide, and Nitrate Ions on Obelisc N
March 3, 2010
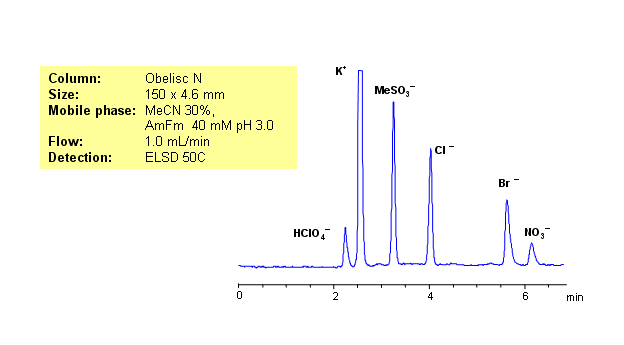
Ion chromatography is usually used for analysis of hydrophilic organic and inorganic ions. Same separation can be achieved on HILIC/mixed-mode Obelisc N HPLC columns. Obelisc N HPLC columns have very polar groups on their surface: one of the groups is basic and the other acidic. In case of low organic concentration, two groups are connected by hydrophilic linker. Obelisc N column can be used as cation-exchange and anion-exchange column. This allows to separate positively and negatively charged molecules in one run. Five anions (chloride, bromide, methanesulfonate, nitrate and perchlorate) along with one cation (sodium) were separated in one run. Method is compatible with ELSD, CAD and LC/MS and can be used for analysis of various hydrophilic and hydrophobic cations and anions in one HPLC run.
| Column | Obelisc N, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Hydrophilic, Ions |
| Analyzing Compounds | Chloride, Nitrate, Chlorate, Bromide, Potassium |
Application Column
Obelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsChloride
Methanesulfonic Acid
Nitrate
Nitric Acid
Potassium

HPLC Separation of Chloride and Nitrate Ions
January 13, 2005
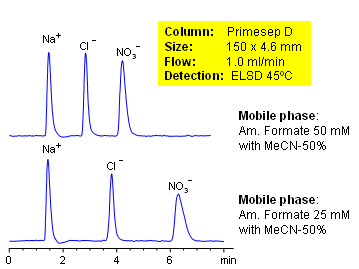
High nitrate concentrations in water and soil can be toxic to humans, fish and domestic animals. Chloride can be used for analysis of water contamination. Primesep D column can be used to separate and quantify both ions. The baseline separation between chloride and nitrate can be increased by decreasing the ammonium formate concentration in the mobile phase. Evaporative Light Scattering Detection (ELSD) used.
| Column | Primesep D, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Hydrophilic, Ion |
| Analyzing Compounds | Chloride, Nitrate |
Application Column
Primesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsNitrate
Nitric Acid
Sodium