| CAS Number | 14265-44-2 |
|---|---|
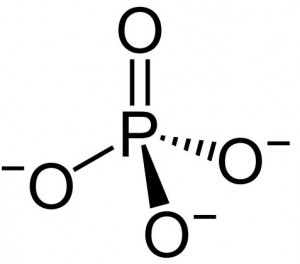
| Molecular Formula | O4P |
| Molecular Weight | 94.971 |
| InChI Key | NBIIXXVUZAFLBC-UHFFFAOYSA-K |
| LogP | -0.770 |
| Synonyms |
|
Applications:
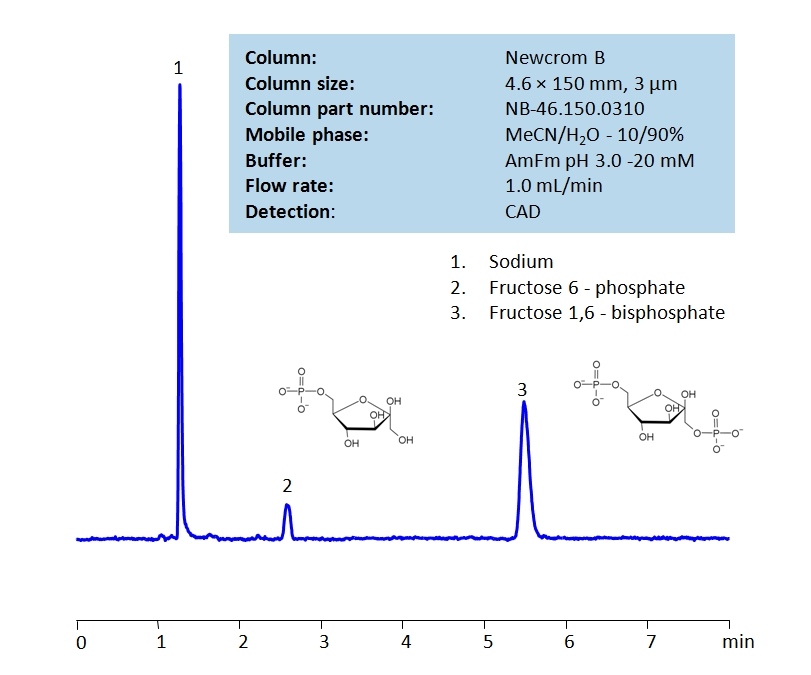
HPLC Determination of Fructose 1,6 Bisphosphate on Newcrom B Column
May 13, 2021
HPLC Method for Fructose 1,6 Bisphosphate, Phosphate, Fructose 6-Phosphate on Newcrom B by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Fructose 1,6 Bisphosphate, Phosphate, Fructose 6-Phosphate.
Fructose 1,6-biphosphate, alternatively known as Harden-Young ester, is the main form glucose and fructose take when they enter cells. It is a key component of the glycolysis metabolic pathway and is the product of the phosphorylation of fructose 6-phosphate. It has the chemical formula C6H14O12P2.
Using a Newcrom B mixed-mode column and a mobile phase consisting of water and acetonitrile with an ammonium formate (AmFm) buffer, Fructose 1,6-bisphosphate can be retained, separated (from fructose 6-phosphate), and measured. Fructose 1,6 bisphosphate can be detected using charged aerosol detection (CAD) with high resolution.
| Column | Newcrom B, 4.6 x 150 mm, 3 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 10/90% |
| Buffer | AmFm – pH 3.0 – 20 mm |
| Flow Rate | 1.0 ml/min |
| Detection | CAD |
| Class of Compounds |
Sugar Phosphate |
| Analyzing Compounds | Fructose 1,6 Bisphosphate, Phosphate, Fructose 6-Phosphate |
Application Column
Newcrom B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 3 µm
Pore Size: 100 A
Column options: dual ended
Fructose 6-Phosphate
Phosphate

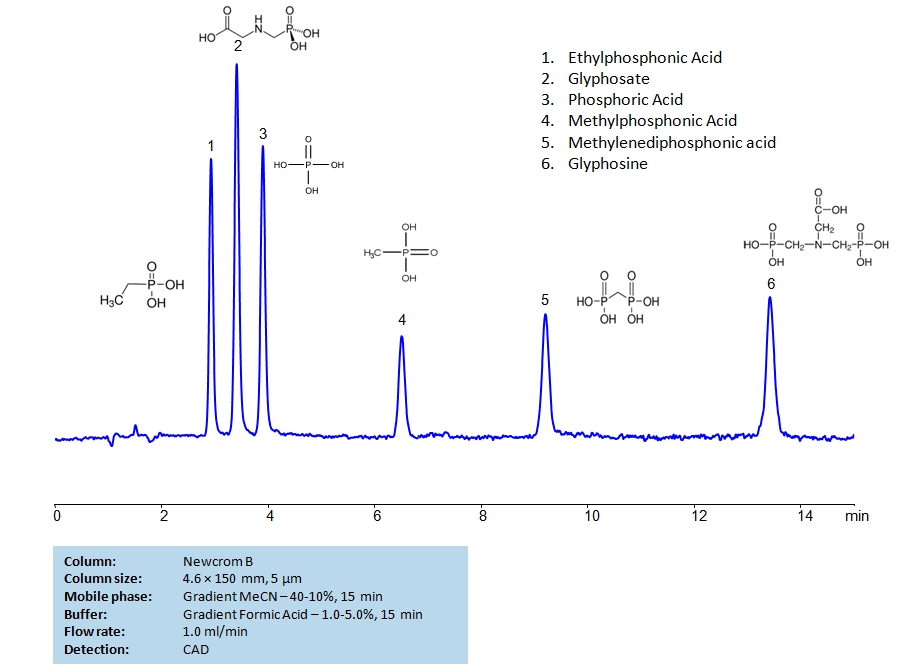
HPLC Separation of Glyphosate, Glyphosine, Ethylphosphonic, Methylphosphonic, Methylenediphosphonic and Phosphoric Acids on Newcrom B Column
December 4, 2019
HPLC Method for Ethylphosphonic Acid, Methylphosphonic Acid, Glyphosate, Glyphosine, Methylenediphosphonic acid, Phosphate, Phosphoric Acid on Newcrom B by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Ethylphosphonic Acid, Methylphosphonic Acid, Glyphosate, Glyphosine, Methylenediphosphonic acid, Phosphate, Phosphoric Acid.
Glyphosate is an herbicide with a chemical formula of C3H8NO5P. It works through blocking enzymes, like 5-enolpyruvylshikimate-3-phosphate synthase, essential for plant growth. It is typically found in agricultural work, but can occasionally be found in forestry and garden care.
Glyphosine is a synthetic plant growth regulator with the chemical formula of C4H11NO8P2. It is a colorless liquid that works through decreasing chlorophyll production.
Ethylphosphonic acid has a C2H5P(O)(OH)2 chemical formula. It is typically found as white crystals or crystalline powder. It is often used as an internal standard when researching fosfomycin in human plasma as well as a synthetic nucleotide analog.
Methylphosphonic acid is an organophosphorus compound with the chemical formula CH3P(OH)2. It is often used in some lubricant additives, textile treatments, and in synthesis of phosphonate compounds, like the previously mentioned Glyphosate.
Methylenediphosphonic acid has the chemical formula CH2[P(O)(OH)2]2. It is typically seen as a precursor in synthesis of Mesoporous aluminum organophosphate, if alkyltrimethylammonium, and Tetraester of methylenediphosphonic acids.
Phosphoric acid is an inorganic compound with chemical formula H3PO4. It is odorless and colorless, which leads to it’s common use in soft drunks to help preserve the product. It is also used in fertilizers, metal treatment, and corrosion inhibition. Excessive intake of it is not recommended.
Ethylphosphonic Acid, Methylphosphonic Acid, Glyphosate, Glyphosine, Methylenediphosphonic acid, Phosphate, Phosphoric Acid can be retained and analyzed using the Newcrom B stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a formic acid buffer. Detection is performed using CAD.
| Column | Newcrom B, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN Gradient -40-10%, 15 min |
| Buffer | Formic Acid Gradient -1- 5%, 15 min |
| Flow Rate | 1.0 ml/min |
| Detection | CAD |
| Class of Compounds | Acids, Plant growth regulator, Herbicide, Hydrophilic, Ionizable |
| Analyzing Compounds | Ethylphosphonic Acid, Methylphosphonic Acid, Glyphosate, Glyphosine, Methylenediphosphonic acid, Phosphate, Phosphoric Acid |
Application Column
Newcrom B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Glyphosate
Glyphosine
Methylenediphosphonic acid
Methylphosphonic Acid
Phosphate
Phosphoric Acid

HPLC Separation of Glyphosate, Ethylphosphonic Acid and Methylphosphonic Acid on Newcrom B Column
December 2, 2019
HPLC Method for Methylphosphonic Acid, Ethylphosphonic Acid, Glyphosate, Phosphate on Newcrom B by SIELC Technologies
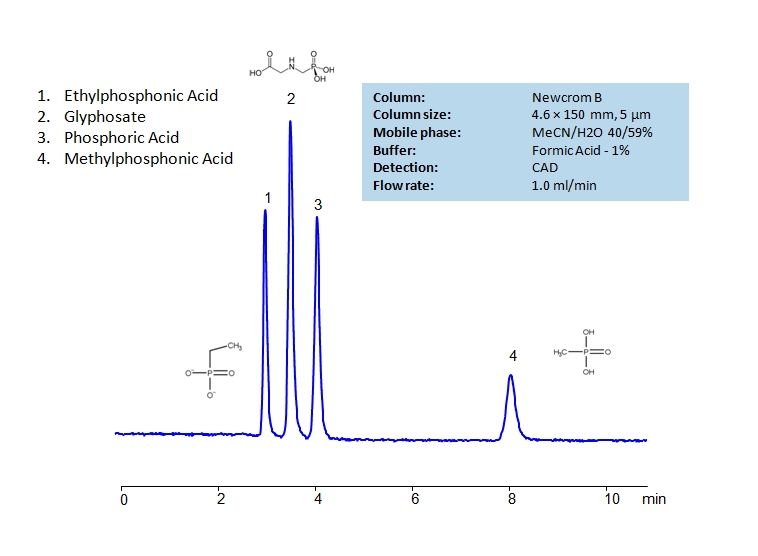 High Performance Liquid Chromatography (HPLC) Method for Analysis of Methylphosphonic Acid, Ethylphosphonic Acid, Glyphosate, Phosphate.
High Performance Liquid Chromatography (HPLC) Method for Analysis of Methylphosphonic Acid, Ethylphosphonic Acid, Glyphosate, Phosphate.
Ethylphosphonic acid has a C2H5P(O)(OH)2 chemical formula. It is typically found as white crystals or crystalline powder. It is often used as an internal standard when researching fosfomycin in human plasma as well as a synthetic nucleotide analog. Glyphosate is an herbicide with a chemical formula of C3H8NO5P. It works through blocking enzymes, like 5-enolpyruvylshikimate-3-phosphate synthase, essential for plant growth. It is typically found in agricultural work, but can occasionally be found in forestry and garden care.
Methylenediphosphonic acid has the chemical formula CH2[P(O)(OH)2]2. It is typically seen as a precursor in synthesis of Mesoporous aluminum organophosphate, if alkyltrimethylammonium, and Tetraester of methylenediphosphonic acids.
Phosphoric acid is an inorganic compound with chemical formula H3PO4. It is odorless and colorless, which leads to it’s common use in soft drunks to help preserve the product. It is also used in fertilizers, metal treatment, and corrosion inhibition. Excessive intake of it is not recommended.
Methylphosphonic Acid, Ethylphosphonic Acid, Glyphosate, Phosphate can be retained and analyzed using the Newcrom B stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a formic acid buffer. Detection is performed using CAD.
| Column | Newcrom BH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 40/59% |
| Buffer | Formic Acid – 1% |
| Flow Rate | 1.0 ml/min |
| Detection | CAD |
| Class of Compounds | Ions, Hydrophilic, Ionizable |
| Analyzing Compounds | Methylphosphonic Acid, Ethylphosphonic Acid, Glyphosate, Phosphate |
Application Column
Newcrom BH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Glyphosate
Methylphosphonic Acid
Phosphate

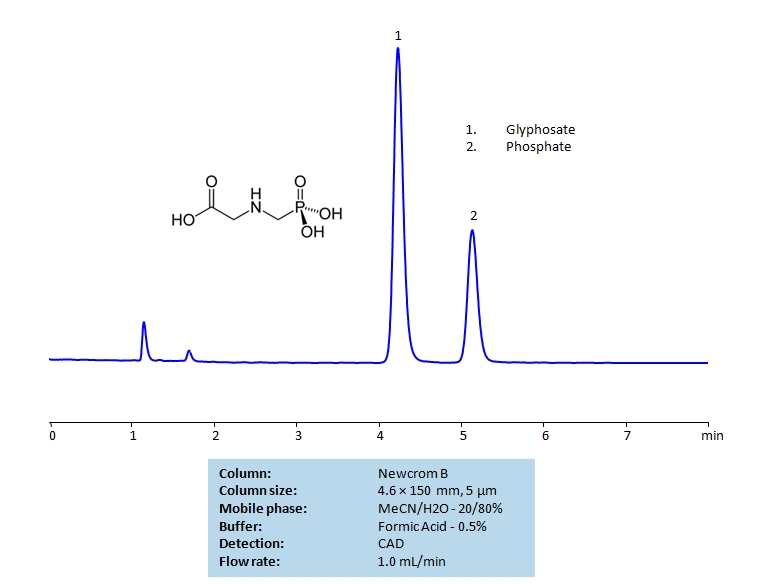
HPLC Separation of Glyphosate and Phosphate Ion on Newcrom B Column
December 2, 2019
HPLC Method for Glyphosate, Phosphate on Newcrom B by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Glyphosate and Phosphate Ion
Glyphosate is a broad-spectrum herbicide which is used to kill weeds. The presence of glyphosate is strongly regulated by various governing agencies in the US, Europe, and Asia. Glyphosate is characterized by its carboxylic acid and phosphate group on either end.
Glyphosate and phosphate ions can be retained and separated with a Newcrom B mixed-mode column using a mobile phase consisting of acetonitrile (MeCN), water, and formic acid (H3PO4) as a buffer. This method is compatible with mass spectrometry and be detected via a Charged aerosol detector (CAD).
| Column | Newcrom B, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 20% |
| Buffer | Formic Acid – 0.5% |
| Flow Rate | 1.0 ml/min |
| Detection | CAD |
| Class of Compounds |
Insecticide, Herbicide, Fungicide, Hydrophobic, Ionizable |
| Analyzing Compounds | Glyphosate, Phosphate |
Application Column
Newcrom B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Phosphate

HPLC Separation of Ethylphosphonic, Methylphosphonic, Methylenediphosphonic and Phosphoric Acids on Newcrom B Column
December 2, 2019
HPLC Method for Ethylphosphonic Acid, Methylphosphonic Acid, Methylenediphosphonic acid, Phosphate, Phosphoric Acid on Newcrom B by SIELC Technologies  High Performance Liquid Chromatography (HPLC) Method for Analysis of Ethylphosphonic Acid, Methylphosphonic Acid, Methylenediphosphonic acid, Phosphate, Phosphoric Acid
High Performance Liquid Chromatography (HPLC) Method for Analysis of Ethylphosphonic Acid, Methylphosphonic Acid, Methylenediphosphonic acid, Phosphate, Phosphoric Acid
Ethylphosphonic acid has a C2H5P(O)(OH)2 chemical formula. It is typically found as white crystals or crystalline powder. It is often used as an internal standard when researching fosfomycin in human plasma as well as a synthetic nucleotide analog. Glyphosate is an herbicide with a chemical formula of C3H8NO5P. It works through blocking enzymes, like 5-enolpyruvylshikimate-3-phosphate synthase, essential for plant growth. It is typically found in agricultural work, but can occasionally be found in forestry and garden care.
Methylphosphonic acid is an organophosphorus compound with the chemical formula CH3P(OH)2. It is often used in some lubricant additives, textile treatments, and in synthesis of phosphonate compounds, like the previously mentioned Glyphosate.
Methylenediphosphonic acid has the chemical formula CH2[P(O)(OH)2]2. It is typically seen as a precursor in synthesis of Mesoporous aluminum organophosphate, if alkyltrimethylammonium, and Tetraester of methylenediphosphonic acids. Ethylphosphonic Acid, Methylphosphonic Acid, Methylenediphosphonic acid, Phosphate, Phosphoric Acid can be retained and analyzed using the Newcrom B stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a formic acid buffer. Detection is performed using CAD.
| Column | Newcrom BH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 20/78% |
| Buffer | Formic Acid – 2% |
| Flow Rate | 1.0 ml/min |
| Detection | CAD |
| Class of Compounds | Acids, Hydrophilic, Ionizable |
| Analyzing Compounds | Ethylphosphonic Acid, Methylphosphonic Acid, Methylenediphosphonic acid, Phosphate, Phosphoric Acid |
Application Column
Newcrom BH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Methylenediphosphonic acid
Methylphosphonic Acid
Phosphate
Phosphoric Acid

HPLC Separation of Inorganic Anions on Newcrom BH Column
October 23, 2019
HPLC Method for Sodium, Phosphate, Chloride, Bromide, Nitrate, Sulfate, Iodine, Perchlorate, Iodide on Newcrom BH by SIELC Technologies
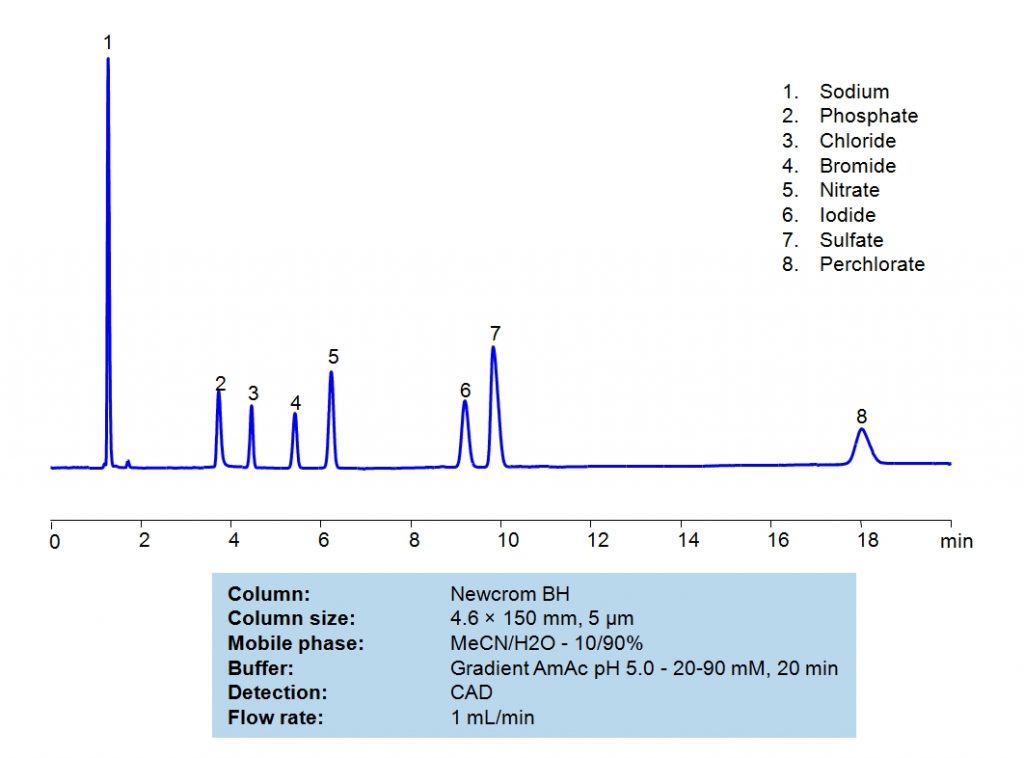
High Performance Liquid Chromatography (HPLC) Method for Analysis of Sodium, Phosphate, Chloride, Bromide, Nitrate, Sulfate, Iodine, Perchlorate, Iodide
Sodium, which has a chemical symbol of Na, is a soft alkali metal that is highly reactive. It is found in abundance in everyday materials like table salt, sea water, and even the Earth’s crust. It is crucial for the body’s function and fluid balance.
Phosphate, PO43-, is a compound that plays an important part in biological energy transfer. It contains phosphorus, which is also a key component in bones and teeth. It is also a buffer, which helps maintain pH levels in a body.
Chloride is typically used to refer to a compound or molecule that contains a chlorine anion: Cl–. It is an electrolyte that is essential for bodily functions including blood pH, fluid balance, cellular functions, and more. Imbalance of chloride can indicate underlying health problems.
Bromide is typically used to refer to a compound or molecule that contains a bromide anion: Br–. It is often found in anticonvulsants, flame-retardant materials, and cell stains.
Nitrate, NO3–, is a compound of nitrogen and oxygen. It is essential as a nutrient in plants; therefore, it is often used in fertilizers. It is easily found in leafy greens. While it is important for cardiovascular health, too high of exposure to it can be dangerous.
Iodide, the Ionic form of Iodine, I– is essential for thyroid hormone production. Lack of it can lead to iodine deficiency disorders. It is typically found in Iodized salt and occasionally as an antiseptic.
Sulfate is an ionic chemical with the formula SO42-. In nature, it is usually found in geodes like gypsum. It is also created in atmosphere from sulfur dioxide. It is used for a variety of applications, but most notably, coloring wood and creating light-reflecting paints.
Perchlorate is an inorganic compound with the formula ClO4–. It is most typically used for explosives. For example, fireworks and rocket propellants. Due to it’s water solubility, it can easily contaminate it. When ingested, it can pose a health risk and cause developmental harm.
Sodium, Phosphate, Chloride, Bromide, Nitrate, Sulfate, Iodine, Perchlorate, Iodide can be retained and analyzed using the Newcrom BH stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a Ammonium Acetate buffer. Detection is performed using CAD.
| Column | Newcrom BH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 10/90% |
| Buffer | Gradient AmAc pH 5.0 – 20-90 mM , 20 min |
| Flow Rate | 1.0 ml/min |
| Detection | CAD (Corona) (MS-compatible mobile phase) |
Application Column
Newcrom BH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Chloride
Iodide
Iodine
Nitrate
Perchlorate
Phosphate
Sodium
Sulfate

HPLC Analysis of Sulfate and Phosphate Ions on Primesep Columns
October 14, 2010
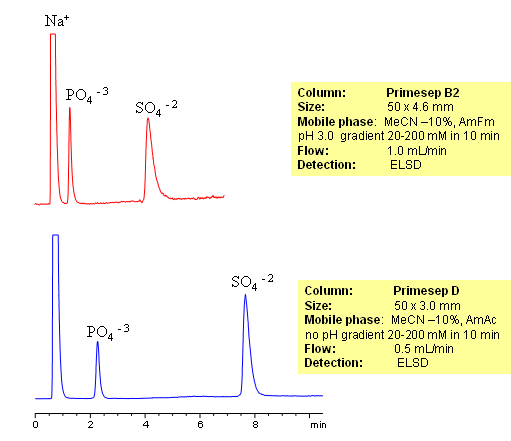
Inorganic and organic ions are usually analyzed by ion-exchange mechanism. In this application, we used short Primesep B2 and Primesep D mixed-mode HPLC columns to retain and separate phosphate and sulfate ions. Both ions are retained by ion-exchange mechanism. Detection technique for analysis of these anions is Evaporative Light-Scattering Detector (ELSD).
Application Column
Primesep B2
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsSulfate

HPLC Separation of Citric Acid and Phosphate Ions in HILIC Chromatography
July 16, 2009
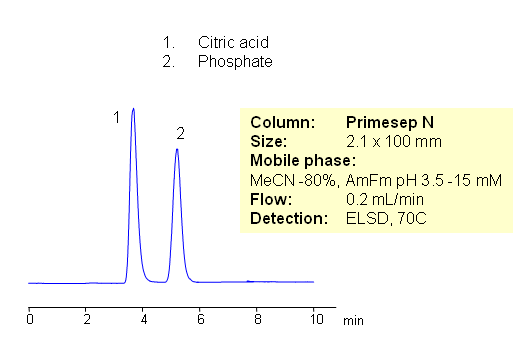
Citric acid and phosphate ion are separated by HILIC on Primesep N HILIC HPLC column. Both compounds are very hydrophilic. Citric acid and phosphate ions are well separated with excellent peak shape. Method can be used for quantitation of citrate and phosphate in various formulations. Because of the lack of UV activity, separation can be monitored by LC/MS, ELSD or Corona CAD. Method can be used for other hydrophilic organic and inorganic acids.
Application Column
Primesep N
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPhosphate

HPLC Separation of Sulfate and Phosphate Ions on Mixed-Mode HPLC
December 6, 2007
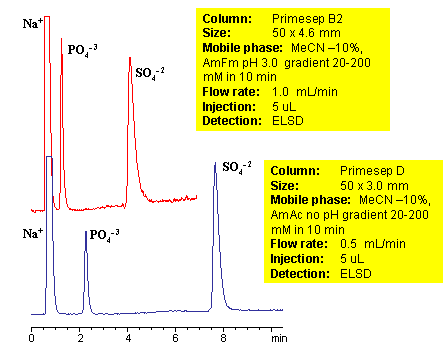
Sulfate and phosphate are separated on Primesep B2 and Primesep D column by anion-exchange mechanism. Because both sulfate and phosphate ions are not UV-active, ELSD is used to monitor separation of both anions. Method can be used to quantitate phosphate and sulfate in various pharmaceutical and chemical formulation, products and solution, drinking and ground water. Retention time is adjusted by increase or decrease of buffer concentrations. Two anions can be retained and separated on a very short column due to strong ion-exchange interaction. Method shows good reproducibility and versatility.
| Column | Primesep B2, 4.6×50 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm pH 3.0 |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Ions, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Sodium, Phosphate, Sulfate |
Application Column
Primesep B2
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsSodium
Sulfate