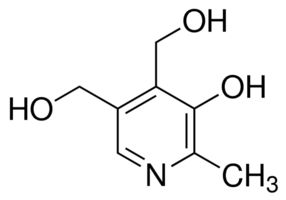
| CAS Number | 65-23-6 |
|---|---|
| Molecular Formula | C8H11NO3 |
| Molecular Weight | 169.180 |
| InChI Key | LXNHXLLTXMVWPM-UHFFFAOYSA-N |
| LogP | -0.770 |
| Synonyms |
|
Applications:
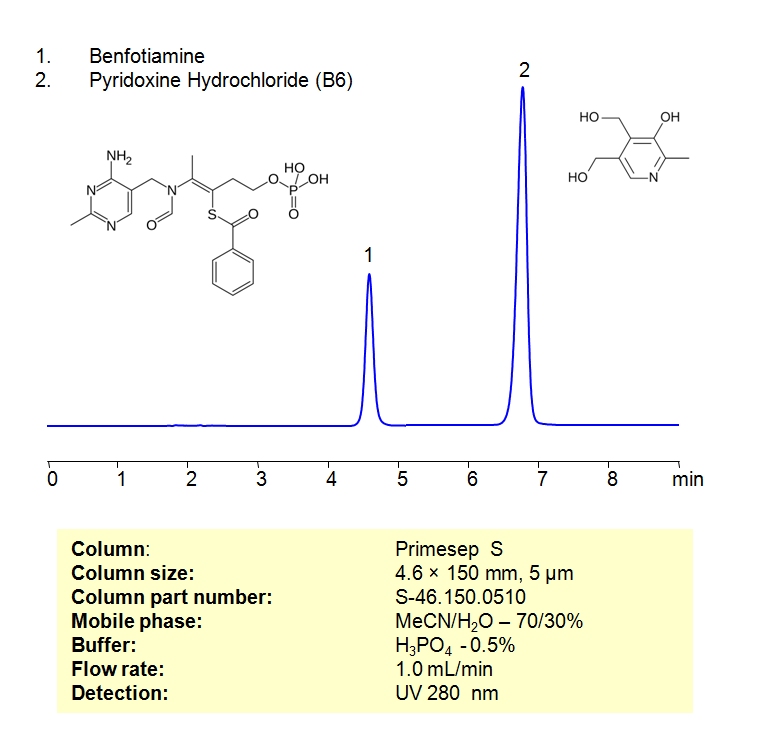
HPLC Separation of Pyridoxine Hydrochloride (Vitamin B6) and Benfotiamine in Milgamma 100 Tablets on Primesep S Column
December 10, 2020
Separation type: Liquid Chromatography Mixed-mode
High Performance Liquid Chromatography (HPLC) Method for Analysis of Pyridoxine Hydrochloride (Vitamin B6) and Benfotiamine
Pyridoxine Hydrochloride (also known as Vitamin B6), is a naturally occurring vitamin that can be found in a wide variety of common foods, such as meat, grains, and avocados. Benfotiamine is a synthetic form of Vitamin B1 that has shown early signs of treating nerve damage, Alzheimer’s, alcohol dependence. They can both be found in Milgamma 100 tablets, a medicine prescribed to treat Vitamin B1 and B6 deficiency.
These two vitamins can be detected in the low UV regime. Using a Primesep S normal-phase column and a mobile phase consisting of water and acetonitrile (MeCN) with a phosphoric acid (H3PO4) buffer, Pyridoxine Hydrochloride and Benfotiamine can be retained, separated, and analyzed. This analysis method can be UV detected at 280 nm.
| Column | Primesep S 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O -70/30%, |
| Buffer | H3PO4 – 0.5% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 280 nm |
| Class of Compounds |
Vitamin, Drug |
| Analyzing Compounds | Pyridoxine Hydrochloride, Benfotiamine |
Application Column
Primesep S
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsBenfotiamine
Pyridoxine hydrochloride
Vitamin B6 (Pyridoxine)

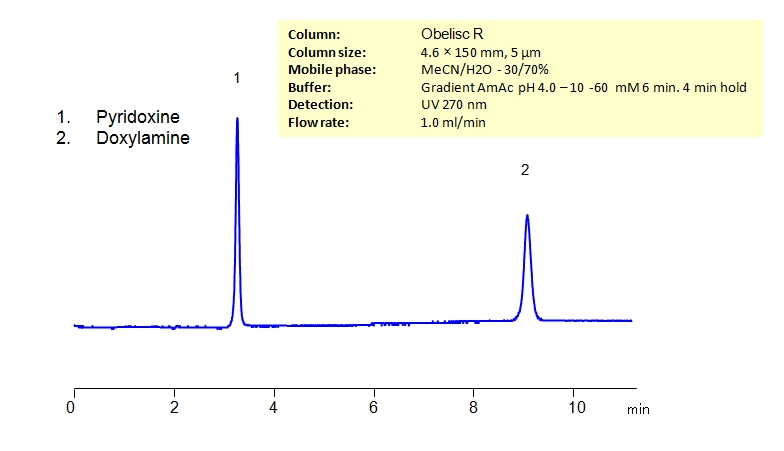
HPLC Method for Separation of Pyridoxine and Doxylamine on Obelisc R Column
March 26, 2020
| Column | Obelisc R, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | AmAc pH 4.0. gradient from 10 to 60 mM in 6 min, 4 min hold |
| Flow Rate | 1.0 ml/min |
| Detection | UV 270 nm, MS- compatible mobile phase |
| Class of Compounds | Hydrophobic, Drug |
| Analyzing Compounds | Pyridoxine, Doxylamine |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsVitamin B6 (Pyridoxine)

HPLC Method for Separation of Pyridoxine and Doxylamine on Primesep S Column
March 26, 2020
Pyridoxine, also known as Vitamin B6, is an essential nutrient required by the body to produce red blood cells and for proper nerve functioning. Doxylamine is a common treatment for insomnia. When taken together, they can treat morning sickness in pregnant women.
Pyridoxine and Doxylamine can be retained, separated, and detected on a normal-phase Primsep S using an isocratic analytical method with a simple mobile phase of water, Acetonitrile, and an Ammonium Formate buffer. This analysis method can be detected in the low UV regime at 270 nm.
| Column | Primesep S, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm pH 3.0- 100 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 270 nm, MS- compatible mobile phase |
| Class of Compounds | Hydrophobic, Drug |
| Analyzing Compounds | Pyridoxine, Doxylamine |
Application Column
Primesep S
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsVitamin B6 (Pyridoxine)

USP Methods for the Analysis of Pyridoxine for the Legacy L1 Column
June 21, 2012
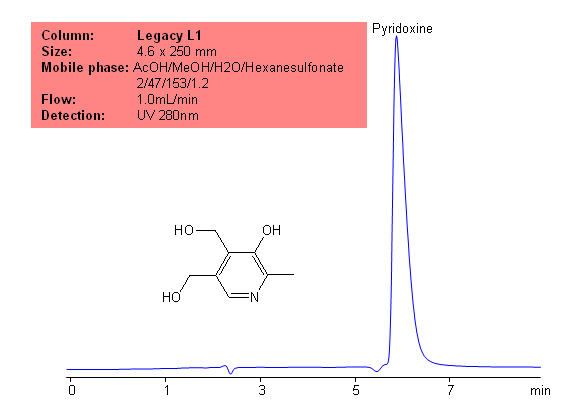
Application Notes: Pyridoxine is part of the vitamin B complex group. Pyridoxine is important in the body’s daily function as it regulates many enzymatic reactions. The USP HPLC method for the separation of pyridoxine was developed on Legacy L1 column according to the US Pharmacopeia methodology. L1 classification is assigned to reversed-phase HPLC column containing C18 ligand. Support for the material is spherical silica gel with particles size 3-10 um and pore size of 100-120A. Resolution between critical pairs corresponds to rules and specifications of UPS.
Application Columns: Legacy L1 C18 HPLC column
Application compounds: Pyridoxine
Mobile phase: AcOH/MeOH/H2O/Hexanesulfonate (2/47/153/1.2)
Detection technique: UV
Reference: USP35- NF30
| Column | Legacy L1, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | AcOH/MeOH/H2O/Hexanesulfonate 2/47/153/1.2 |
| Buffer | Hexanesulfonate |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 280 nm |
| Class of Compounds |
Drug, Vitamin B₆, Hydrophobic, Ionizable |
| Analyzing Compounds | Pyridoxine |
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select optionsVitamin B6 (Pyridoxine)

HPLC Analysis of Active Drug in a Formulation
October 4, 2010
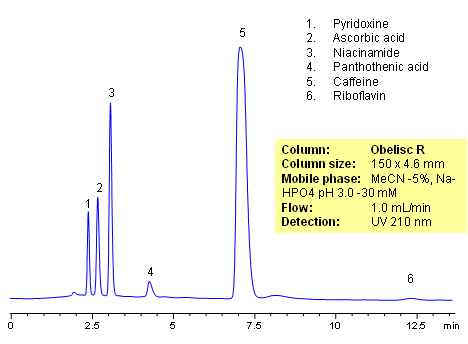
HPLC method for separation of active ingredients of drug/supplemental composition was developed on an Obelisc R trimodal HPLC column. Compounds are retained by combination of reversed-phase, cation-exchange and anion-exchange mechanisms. Compounds are well separated, and method can be used for quantitation of pyridoxine, ascorbic acid, niacinamide, pantothenic acid, caffeine and riboflavin in a mixture or as separate compounds in various complex mixtures. Various detection techniques can be applied for quantitation (ELSD, UV, LC/MS, Corona). This HPLC method can be adopted as general approach for analysis of active drug components in various formulations.
| Column | Obelisc R , 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O -5/95% |
| Buffer | NaHPO4 pH 3.0 – 30 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Vitamin B₆, Hydrophobic, Ionizable |
| Analyzing Compounds | Pyridoxine, Ascorbic acid, Niacinamide, Pantothenic acid, Caffeine, Riboflavin |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsAscorbic Acid
Caffeine
Niacinamide
Pantothenic Acid
Vitamin B6 (Pyridoxine)

HPLC Separation of Vitamin C, Vitamin Group B, and Related Impurities
August 22, 2008
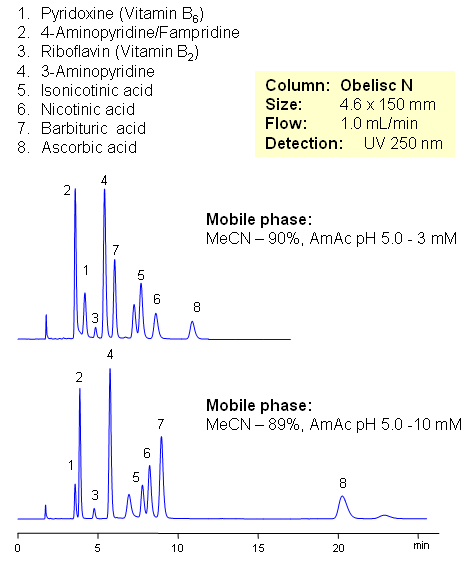
Vitamin C (ascorbic acid) and Vitamins Group B are separated on Obelisc N mixed-mode column. Method can be used in quantitation and determination of polar vitamins in various formulations and dietary supplements. HPLC method can be based on UV, Evaporative Light Scattering Detection (ESLD), RI or MS detection. Effect of sample matrix can be eliminated by changing mobile phase conditions. Buffer concentration, buffer pH and amount of ACN will affect every vitamin differently due to difference in polar and ionic properties.
| Column | Obelisc N , 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc pH 5.0 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Vitamin B₆, Hydrophobic, Ionizable |
| Analyzing Compounds | Pyridoxine, Ascorbic acid, Niacinamide, Pantothenic acid, Caffeine, Riboflavin, Barbituric Acid, 3- Aminopyrine |
Application Column
Obelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select options3-Aminopyridine
4-Aminopyridine/Fampridine
Ascorbic Acid
Barbituric Acid
Isonicotinic Acid
Nicotinic Acid/Niacin (3-pyridinecarboxylic acid)
Vitamin B2 (Riboflavin)
Vitamin B6 (Pyridoxine)

HILIC Separation of Vitamins Group B
August 22, 2008
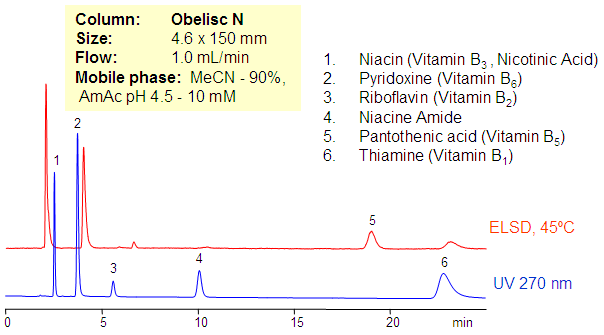
Separation of vitamins group B is achieved on Obelisc N column in HILIC mixed-mode. Vitamins of this group are different in polarity and ionic properties. Retention and separation is achieved by optimization of amount of ACN, buffer and buffer pH. Combination of UV and ELSD detection is used to monitor HPLC separation. B vitamins are water-soluble vitamins that play an important role in cell metabolism. Supplements containing all six are generally referred to as a vitamin B complex. Individual B vitamin supplements are referred to by the specific name of each vitamin. This method can be used to analyze individual B vitamins as well as vitamin B complex. Isolation of impurities as well as degradation products is possible by preparative chromatography.
| Column | Obelisc N , 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAC pH 4.5 – 10 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm, ELSD |
| Class of Compounds |
Drug, Vitamin B₆, Hydrophobic, Ionizable |
| Analyzing Compounds | Pyridoxine, Niacinamide, Pantothenic acid, Riboflavin |
Application Column
Obelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsVitamin B1 (Thiamine)
Vitamin B2 (Riboflavin)
Vitamin B3 (Niacin)
Vitamin B5 (Pantothenic Acid)
Vitamin B6 (Pyridoxine)
UV Detection

HPLC Separation of Vitamin B
April 3, 2004
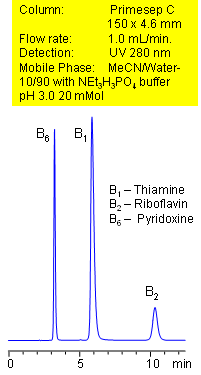
Primesep C separates B vitamins with baseline resolution by a combination of cation exchange, complex formation, and hydrophobic interactions. Vitamin B1 (thiamine), vitamin B2 (riboflavin), and vitamin B6 (pyridoxine) are separated with a mobile phase of water, acetonitrile (MeCN, ACN) and triethylamine (TEA) phosphate with UV detection at 280 nm.
| Column | Primesep C , 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O -10/90% |
| Buffer | NEt3H3PO4 Ph 3.0 – 20 Mm |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 280 nm |
| Class of Compounds |
Drug, Vitamin B₆, Hydrophobic, Ionizable |
| Analyzing Compounds | Pyridoxine, Thiamine, Riboflavin |
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsVitamin B1 (Thiamine)
Vitamin B2 (Riboflavin)
Vitamin B6 (Pyridoxine)
Vitamins