| CAS Number | 63-74-1 |
|---|---|
| Molecular Formula | C6H8N2O2S |
| Molecular Weight | 172.202 |
| InChI Key | FDDDEECHVMSUSB-UHFFFAOYSA-N |
| LogP | -0.6 |
| Synonyms |
|
Applications:
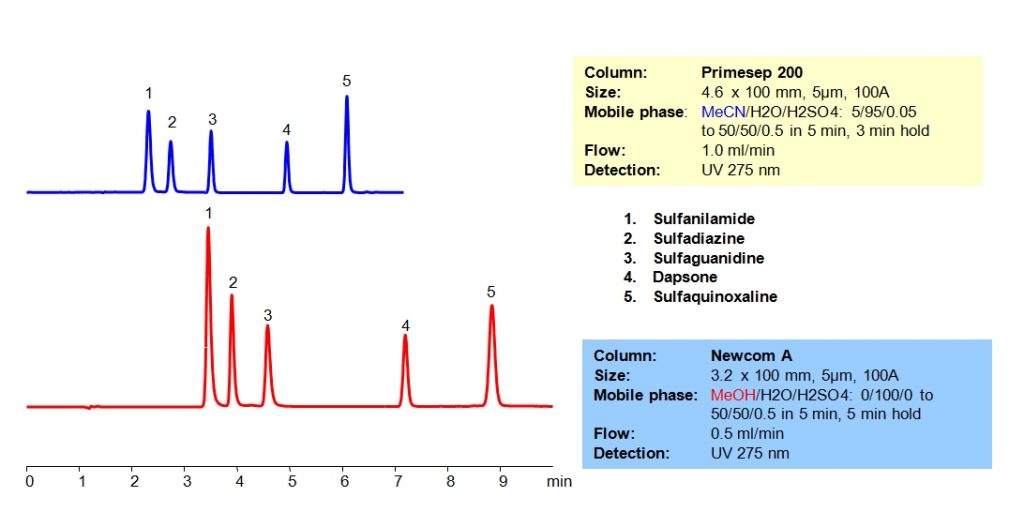
HPLC Separation of Antibiotics on Newcrom A Column
June 11, 2020
HPLC Method for Sulfanilamide, Sulfadiazine, Dapsone, Sulfaguanidine, Sulphaquinoxaline on Newcrom A by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Sulfanilamide, Sulfadiazine, Dapsone, Sulfaguanidine, Sulphaquinoxaline.
Antibiotics are widely used for treatment and prevention of bacterial infections.
Sulfanilamide is a sulfonamide antibacterial drug with the chemical formula C6H8N2O2S. It’s height of use was during World War II to treat and prevent the spread of infections among the Allies. Due to later discovery of more effective antibiotics, it is no longer as widely used. You can find detailed UV spectra of Sulfanilamide and information about its various lambda maxima by visiting the following link.
Sulfadiazine is a sulfonamide antibiotic with the chemical formula C10H10N4O2S. It is the treatment of choice for toxoplasmosis and is considered the second-line treatment against numerous other infections. It works by blocking the synthesis of folic acid in bacteria, which prevents cell reproduction. Sulfadiazine can be either taken orally or applied topically. You can find detailed UV spectra of Sulfadiazine and information about its various lambda maxima by visiting the following link.
Sulfaguanidine is a sulfonamide antibiotic with the chemical formula C7H10N4O2S. It is a guanidine derivative of sulfanilamide that works through inhibiting the synthesis of folic acid in bacteria. Most often, it is used to treat Bacillary dysentery.
Dapsone is a sulfone antibiotic with anti-inflammatory properties. As a gel, it is sold under the brand name Aczone as acne treatment, but it can also be used as part of treatment for other skin conditions including leprosy and dermatitis herpetiformis. It’s chemical formula is C12H12N2O2S.
Sulfaquinoxaline is a sulfonamide antibiotic that is typically used in veterinary medicine. It is used to treat Coccidiosis in cattle and sheep, as well as a variety infections in poultry. It is deemed not suitable for human use. It’s chemical formula is C14H12N4O2S.
Various antibiotics, particularly those with a sulfanilamide structure, were separated in HPLC using mixed-mode columns with varying strengths of ion-pairing groups. Methanol can be used in the mobile phase on Newcrom A column. The antibiotics were resolved on both columns with a gradient mobile phase consisting of acetonitrile (ACN) or methanol (MeOH), water and sulfuric acid (H2SO4) buffer. UV detection at 275nm.
| Column | Newcrom A, 3.2 x 100 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeOH Gradient |
| Buffer | H2SO4 Gradient |
| Flow Rate | 0.5 ml/min |
| Detection | UV 275 nm |
| Class of Compounds | Drugs, Antibiotics |
| Analyzing Compounds | Sulfanilamide, Sulfadiazine, Dapsone, Sulfaguanidine, Sulphaquinoxaline |
Application Column
Newcrom A
Column Diameter: 3.2 mm
Column Length: 100 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Sulfadiazine
Sulfaguanidine
Sulfanilamide
Sulphaquinoxaline

HPLC Separation of Antibiotics on Primesep 200 Column
June 6, 2020
HPLC Method for Sulfanilamide, Sulfadiazine, Sulfaguanidine, Dapsone on Primesep 200 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Sulfanilamide, Sulfadiazine, Sulfaguanidine, Dapsone.
Sulfanilamide is a sulfonamide antibacterial drug with the chemical formula C6H8N2O2S. It’s height of use was during World War II to treat and prevent the spread of infections among the Allies. Due to later discovery of more effective antibiotics, it is no longer as widely used. You can find detailed UV spectra of Sulfanilamide and information about its various lambda maxima by visiting the following link.
Sulfadiazine is a sulfonamide antibiotic with the chemical formula C10H10N4O2S. It is the treatment of choice for toxoplasmosis and is considered the second-line treatment against numerous other infections. It works by blocking the synthesis of folic acid in bacteria, which prevents cell reproduction. Sulfadiazine can be either taken orally or applied topically. You can find detailed UV spectra of Sulfadiazine and information about its various lambda maxima by visiting the following link.
Sulfaguanidine is a sulfonamide antibiotic with the chemical formula C7H10N4O2S. It is a guanidine derivative of sulfanilamide that works through inhibiting the synthesis of folic acid in bacteria. Most often, it is used to treat Bacillary dysentery.
Dapsone is a sulfone antibiotic with anti-inflammatory properties. As a gel, it is sold under the brand name Aczone as acne treatment, but it can also be used as part of treatment for other skin conditions including leprosy and dermatitis herpetiformis. It’s chemical formula is C12H12N2O2S.
Sulfaquinoxaline is a sulfonamide antibiotic that is typically used in veterinary medicine. It is used to treat Coccidiosis in cattle and sheep, as well as a variety infections in poultry. It is deemed not suitable for human use. It’s chemical formula is C14H12N4O2S.
Antibiotics are widely used for treatment and prevention of bacterial infections. Various antibiotics, particularly those with a sulfanilamide structure, were separated in HPLC using mixed-mode columns with varying strengths of ion-pairing groups. Primesep 200 has weak acidic ion-exchange pairing groups while Newcrom A has strong acidic ion-exchange groups. In addition, methanol can be used in the mobile phase on Newcrom A column. The antibiotics were resolved on both columns with a gradient mobile phase consisting of acetonitrile (ACN) or methanol (MeOH), water and sulfuric acid (H2SO4) buffer. UV detection at 275nm.
| Column | Primesep 200, 4.6 x 100 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN Gradient |
| Buffer | H2SO4 – 0.5% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 275 nm |
| Class of Compounds | Drugs, Antibiotics |
| Analyzing Compounds | Sulfanilamide, Sulfadiazine, Sulfaguanidine, Dapsone |
Application Column
Primesep 200
Column Diameter: 4.6 mm
Column Length: 100 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Sulfadiazine
Sulfaguanidine
Sulfanilamide

HPLC Separation of Benzenesulfonamide and Sulfanilamide
August 22, 2008
| Column | Newcrom А, 3.2×50 mm, 3 µm, 100A |
| Mobile Phase | MeOH/H2O – 80/20% |
| Buffer | H2SO4 – 0.005% |
| Flow Rate | 0.5 ml/min |
| Detection | 275 nm |
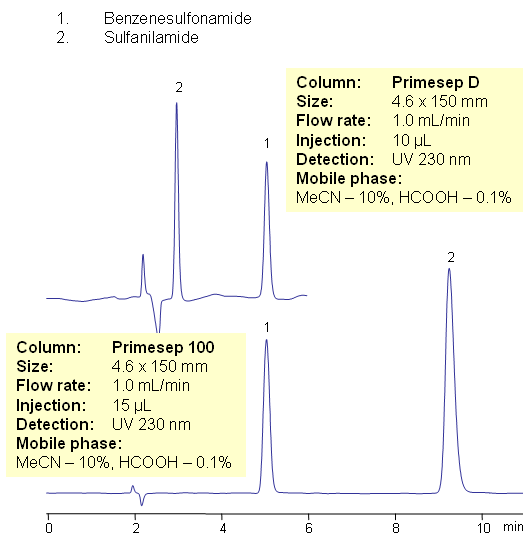
| Column | Primesep D, , Primesep 100 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 10/90% |
| Buffer | Formic Acid – 0.1% |
| Flow Rate | 0.5 ml/min |
| Detection | UV 230 nm |
Both Benzenesulfonamide and sulfanilamide are organic antibacterial compounds differing only in the addition of an extra amino group in sulfanilamide. The closely related compounds can be separated on a mixed-mode Primesep D column by hydrophobic and anion-exchange mechanisms or Primesep 100 column by hydrophobic and cation-exchange mechanisms with the elution order reversed using identical mobile phases. UV Detection at 230nm.
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsSulfanilamide

HPLC Separation of Aromatic Sulfonamides and Hydrozine
December 6, 2007
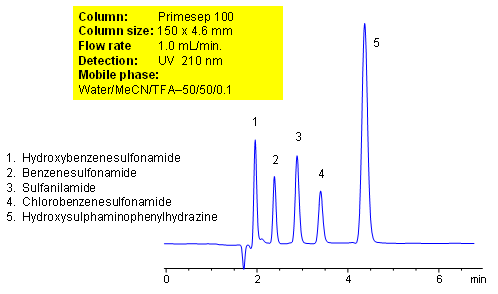
Chlorobenzenesulfonamide
Hydroxybenzenesulfonamide
Hydroxysulphaminophenylhydrazine
Sulfanilamide

Separation of Sulfonamides and Phenylhydrazine by Mixed-Mode HPLC
May 5, 2005
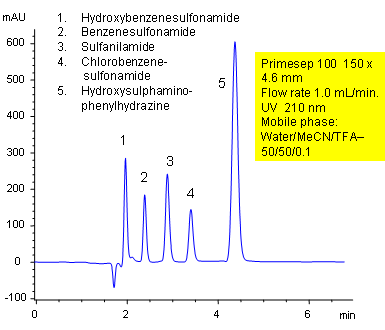
Primesep 100 separates a mixture of sulfonamides (benzenesulfonamide, chlorobenzenesulfonamide, hydroxybenzenesulfonamide) and hydroxysulphaminophenylhydrazine by a mixture of polar and hydrophobic interactions. The stationary phase’s hydrophobic functionality provides a reversed-phase mechanism while the embedded cation-exchange group provides polar interactions. The separation method uses a mobile phase mixture of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) with UV detection at 210 nm. This method is mass spec (LC/MS) and evaporative light scattering (ELSD) compatible.
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsChlorobenzenesulfonamide
Hydroxybenzenesulfonamide
Hydroxysulphaminophenylhydrazine
Sulfanilamide
Sulfonamides