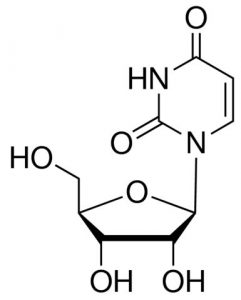
| CAS Number | 58-96-8 |
|---|---|
| Molecular Formula | C9H12N2O6 |
| Molecular Weight | 244.203 |
| InChI Key | DRTQHJPVMGBUCF-XVFCMESISA-N |
| LogP | -2.00 |
| Synonyms |
|
Applications:
HPLC Separation of Nucleosides and Deoxynucleosides
July 23, 2012
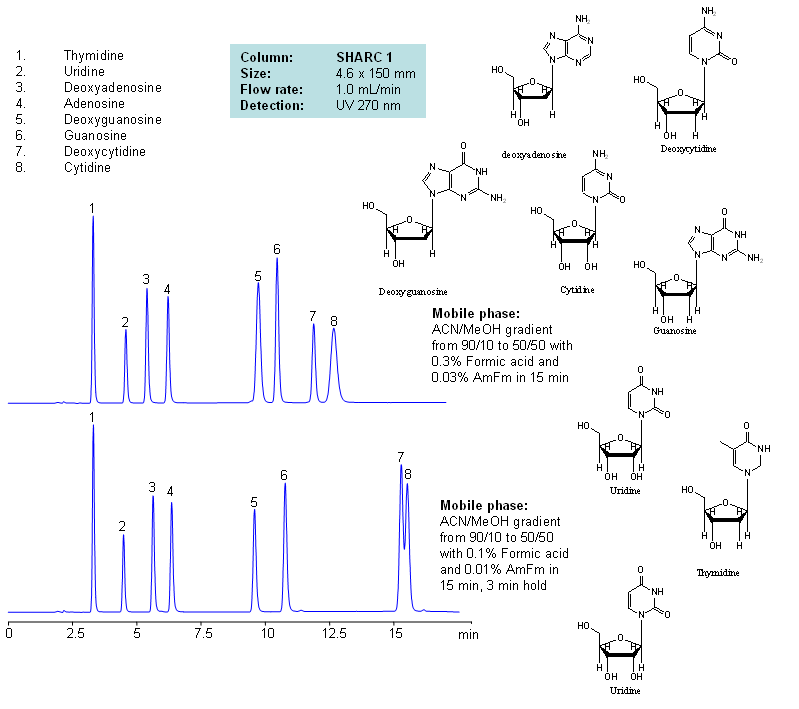
| Column | Sharc 1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH |
| Buffer | AmFm, Formic acid |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Thymidine, Uridine, Deoxyadenosine, Adenosine, Deoxyguanosine, Guanosine, Deoxycytidine, Cytidine |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select optionsCytidine
Deoxyadenosine
Deoxycytidine
Deoxyguanosine
Guanosine
Thymidine
Uridine

HPLC Separation of Thymidine, Uridine, Adenosine, Guanosine, and Cytidine Using the Hydrogen Bonding Method
June 15, 2012
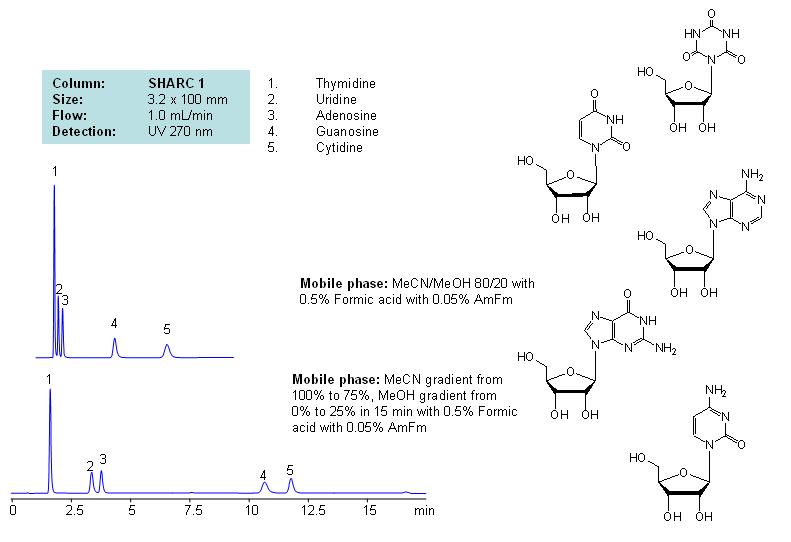
Application Notes: Nucleosides are glycosylamines consisting of nucleobase linked to ribose or deoxyribose sugar and are building blocks for DNA and RNA. These compounds are very polar and contain groups available for hydrogen bonding interaction. Thymidine, uridine, adenosine, guanosine and cytidine were separated using a hydrogen-bonding method. There is a strong correlation between the retention time and mobile phase composition. The strength of hydrogen-bonding interaction increases as the number of hydroxyls in the analytes increase. Additionally the order of elution for compounds depends on the ratio of the mobile phases: acetonitrile and methanol. Our method is compatible with LC/MS and preparative chromatography.
Application Columns: SHARC 1, 3.2×100 mm, 5 um, 100A, To learn more about SHARC 1 columns click here. To order this column click here. To see more chromatographic separations check our web site.
Application Compounds: Thymidine, uridine, adenosine, guanosine and cytidine
| Column | Sharc 1, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH |
| Buffer | AmFm, Formic acid |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Thymidine, Uridine, Adenosine, Guanosine, Cytidine |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select optionsCytidine
Guanosine
Thymidine
Uridine

HPLC Separation of Uracil and Uridine in HILIC Mode on Primesep S2 Column
July 14, 2011
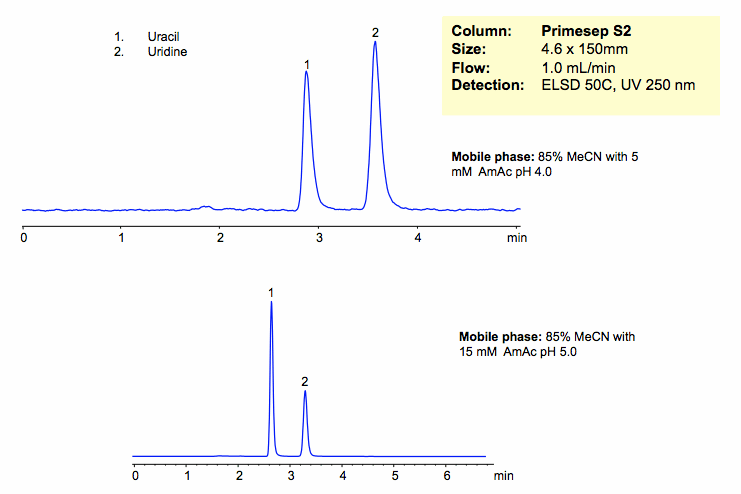
Uracil and uridine are separated based on their polarity. The mechanism of retention is pure HILIC mode. Retention time is controlled by varying the amount of ACN. Buffer concentration and pH will affect ionization and polarity of the stationary phase, and thus indirectly affect retention of neutral compounds. Column is compatible with all general detection techniques.
| Column | Primesep S2, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 80/20% |
| Buffer | AmAc |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD, 50C UV 250 nm |
| Class of Compounds |
Nucleosides, Hydrophilic, Ionizable |
| Analyzing Compounds | Uracil, Uridine |
Application Column
Primesep S2
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsUridine
UV Detection

HPLC Separation of Uridine and Uracil
November 21, 2010
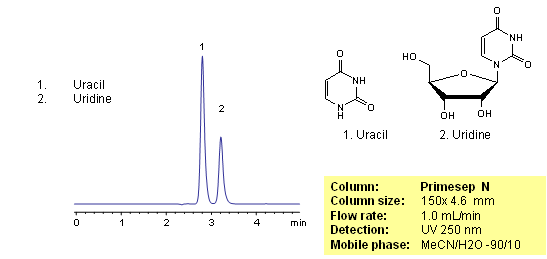
Uracil is a naturally occurring pyrimidine derivative. Uracil is used for drug delivery and as a pharmaceutical. Uridine is a product of uracil and sugar ribose. Uridine is one of components of ribonucleic acid. Uracil and uridine are slightly basic in nature. Both compounds are hydrophilic and uracil is very often is used as a void marker in reversed-phase chromatography. Uracil and uridine are separated based on their polar properties on a Primesep N HILIC column. Uracil and uridine are monitored by UV.
| Column | Primesep N, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 90/10% |
| Buffer | No |
| Flow Rate | 1.0 ml/min |
| Detection | UV 250 nm |
| Class of Compounds |
Nucleosides, Hydrophilic, Ionizable |
| Analyzing Compounds | Uracil, Uridine |
Application Column
Primesep N
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsUridine

HILIC Retention of Uridine and Uracil on Sielc’s HILIC Columns
August 22, 2008
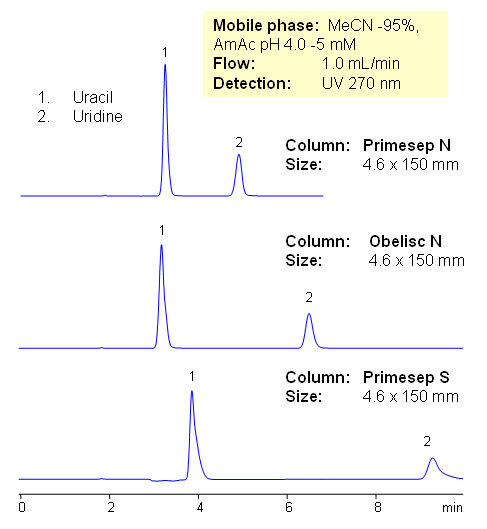
Uridine and uracil are separated on three HILIC columns from SIELC. Primesep N, Primesep S, and Obelisc N columns all have different polarity and different embedded acidic groups on the surface of silica gel: Primesep N is a normal-phase HPLC column with embedded acidic groups with a pKa of about 5. Primesep S is a normal-phase HPLC column with embedded acidic groups with a pKa of about 3. The Primesep S stationary phase retains basic compounds by cation-exchange at pH > 3.
In traditional HILIC mode, a charged or neutral polar analyte interacts with a water layer on the polar stationary phase surface. On Obelisc N the charges are greatly separated and independently accessible, resulting in different selectivity compared to traditional HILIC and silica columns. Mobile phase composition changes the conformation of the long hydrophilic chain. All three columns can be used to analyze uridine and uracil by HPLC with UV, LC/MS and ELSD detection.
| Column | Primesep S, Primesep N, Obelisc N, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 95/5% |
| Buffer | AmAc pH 4.0 – 5mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 270 nm |
| Class of Compounds |
Nucleosides, Hydrophilic, Ionizable |
| Analyzing Compounds | Uracil, Uridine |
Application Column
Primesep S
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep N
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsObelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsUridine

HPLC Separation of Nucleic Bases at pH 4 and 5 on Obelisc N
March 3, 2007
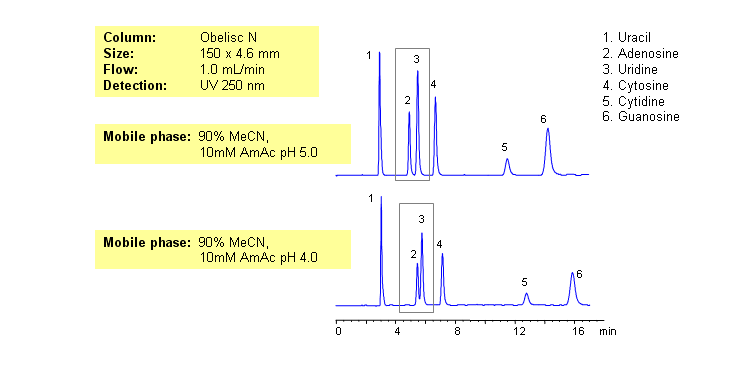
Nucleic bases are biological compounds found in genetic molecules (DNA, RNA). They can be separated on an Obelisc N column, which offers very polar characteristics and can be used with positively or negatively charged groups. Closely-eluted adenosine and uridine can be further separated by simply adjusting the pH of the mobile phase. Mobile phase is water and acetonitrile (MeCN, ACN) with Ammonium Acetate as buffer. UV detection at 250nm.
| Column | Obelisc N, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN -90% |
| Buffer | AmAc |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Uracil, Uridine, Adenosine, Guanosine, Cytidine, Cytosine |
Application Column
Obelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsCytidine
Cytosine
Guanosine
Uracil
Uridine