| Method Development Based on Compound Properties |
 |
By clicking through this interactive guide of analyte properties, you will find an example of separation that can act as a starting point in your method development process.
All major classes of compounds can be analyzed using this Primesep column platform. Keep in mind that there is more than one way to achieve retention and separation of a particular class of compounds. It comes in handy when more than one class of compounds is present in a mixture. This guide will present all available options for each individual class of compound, and help you to find a common method which will work across several compounds classes.
|
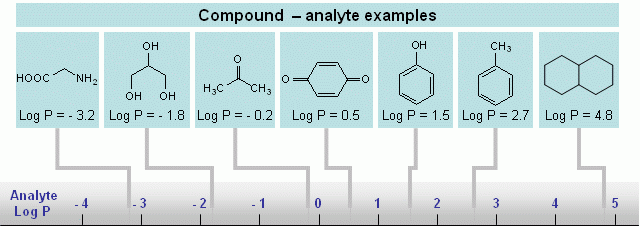
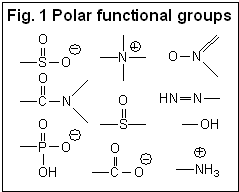
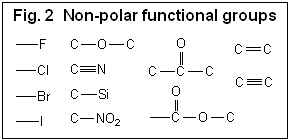
Hydrophilic compounds are compounds with a better solubility in water than in organic solvents, or completely non-soluble in organic solvents (et. acetonitrile, ethanol, hexane). Examples of such compounds are amino acids, sugars, peptides, nucleotides, protonated amines and any other molecules with one or several polar groups (Fig. 1). Not every functional group makes the molecule hydrophilic. The presence of some functional groups (Fig. 2) does not make a molecule significantly polar in terms of LC hydrophobic interaction; there is a more objective characterization of hydrophobic/hydrophilic properties. Log P is a factor which represents the polar properties of the molecules. It is defined as a logarithm of the distribution coefficient of the compound in the liquid/liquid extraction system of water and octanol. The higher the Log P value, the more hydrophobic the compound. Generally, compounds with logP > 1 are well suited for reverse phase chromatography and can be called hydrophobic. LogP for ionizable compounds is pH dependent. If the ionization state of the molecule is changed by the solvent’s pH, then logP will be changed as well. The general rule is that lowering the pH increases logP for acidic compounds, and decreases logP for basic compounds. Many basic compounds become very polar at a low pH, and thus non-suitable for reverse phase separation. Acidic compounds, on the other hand, become more hydrophobic and retain better. |
|
 |
|
 |
 |
