|
Unique elution order of positively charged molecules in a chelating HPLC system
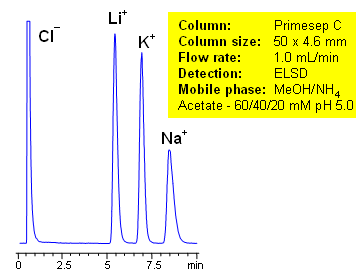
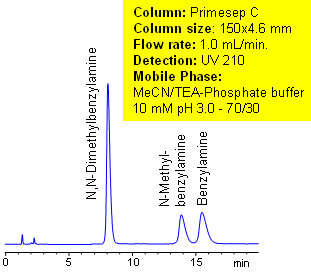
The unique complex properties of the column can be observed in the separation of alkali metals ions. The ions elute on the Primesep C column in reverse order compared to ion-exchange elution. The unusual elution order is found in primary, secondary, and tertiary amines. Secondary and tertiary amines have higher pKa values than primary amines, which makes them retain longer by ion-exchange mechanism. With the complex forming properties of Primesep C columns, the primary amines are more retainable than secondary and tertiary amines. This extraordinary elution pattern allows tailoring the separation of amines to provide the most appropriate peak order for quantitation and preparative chromatography.
|

© SIELC Technologies. 2002 - 2026
Sign up to our newsletter
Contact
Address: 804 Seton Court, Wheeling, IL USA 60090
Tel: (847) 229-2629 | Fax: (847) 655-6079
Sales, Refund and Returns Policy
Email: mail@sielc.com | Sitemap
Host a Customized Seminar at Your Company
Delivering tailored seminars directly to your team.