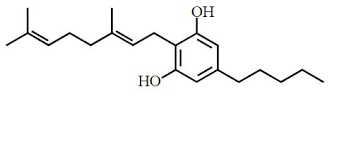
| CAS Number | 25654-31-3 |
|---|---|
| Molecular Formula | C21H32O2 |
| Molecular Weight | 316.5 |
| InChI Key | QXACEHWTBCFNSA-SFQUDFHCSA-N |
| LogP | 312.5 |
| Synonyms |
|
Applications:
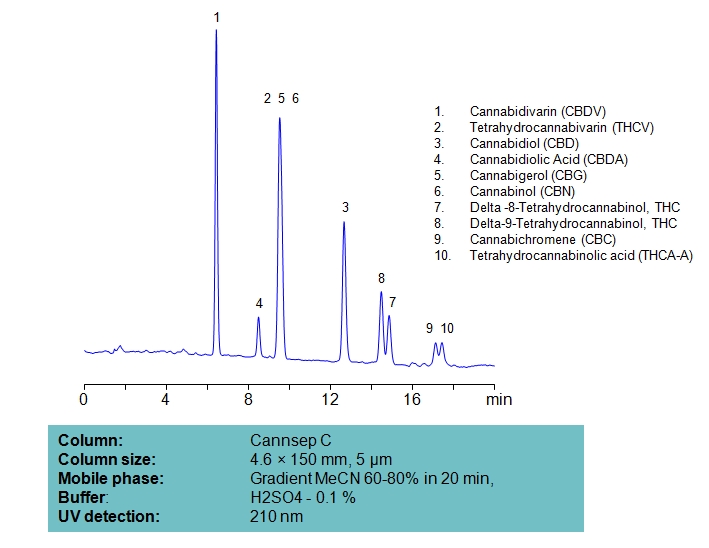
HPLC Separation of Ten Cannabinoids on Cannsep C Column
January 13, 2020
| Column | Cannsep C, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN 60-80% , 20 min |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Hydrophilic, Ionizable, Supplements |
| Analyzing Compounds | Cannabidivarin (CBDV), Tetrahydrocannabivarin (THCV), Cannabidiol (CBD), Delta-9-Tetrahydrocannabinol (THC), Cannabigerol (CBG), Cannabichromene (CBC), Tetrahydrocannabinolic acid (THCA-A) |
Application Column
Cannsep C
The Cannsep family of columns is a specially developed line of reverse-phase columns designed to retain and separate most of the compounds responsible for cannabis's physiological properties. With three options available - Cannsep A, Cannsep B, and Cannsep C - customers can resolve all the various cannabinoids, each providing unique and significantly orthogonal selectivity.
Select optionsCannabidiol (CBD)
Cannabidiolic acid (CBDA)
Cannabidivarin (CBDV)
Cannabigerol (CBG)
Cannabinol (CBN)
Delta-9-Tetrahydrocannabinol (THC)
Tetrahydrocannabinol (THC)
Tetrahydrocannabinolic acid (THCA-A)
Tetrahydrocannabivarin (THCV)

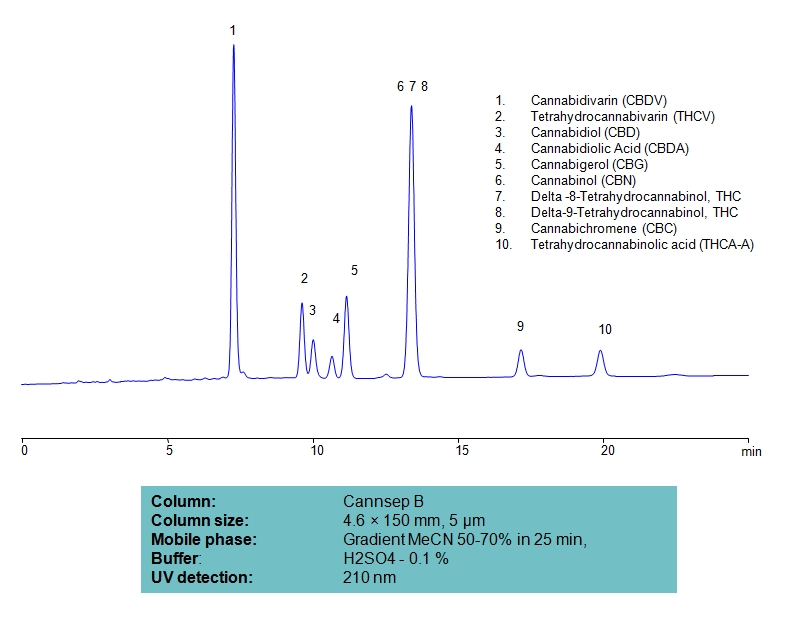
HPLC Separation of Ten Cannabinoids on Cannsep B Column
January 13, 2020
| Column | Cannsep B, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN 50-70% , 25 min |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Hydrophilic, Ionizable, Supplements |
| Analyzing Compounds | Cannabidivarin (CBDV), Tetrahydrocannabivarin (THCV), Cannabidiol (CBD), Delta-9-Tetrahydrocannabinol (THC), Cannabigerol (CBG), Cannabichromene (CBC), Tetrahydrocannabinolic acid (THCA-A) |
Application Column
Cannsep B
The Cannsep family of columns is a specially developed line of reverse-phase columns designed to retain and separate most of the compounds responsible for cannabis's physiological properties. With three options available - Cannsep A, Cannsep B, and Cannsep C - customers can resolve all the various cannabinoids, each providing unique and significantly orthogonal selectivity.
Select optionsCannabidiol (CBD)
Cannabidiolic acid (CBDA)
Cannabidivarin (CBDV)
Cannabigerol (CBG)
Cannabinol (CBN)
Delta-9-Tetrahydrocannabinol (THC)
Tetrahydrocannabinol (THC)
Tetrahydrocannabinolic acid (THCA-A)
Tetrahydrocannabivarin (THCV)

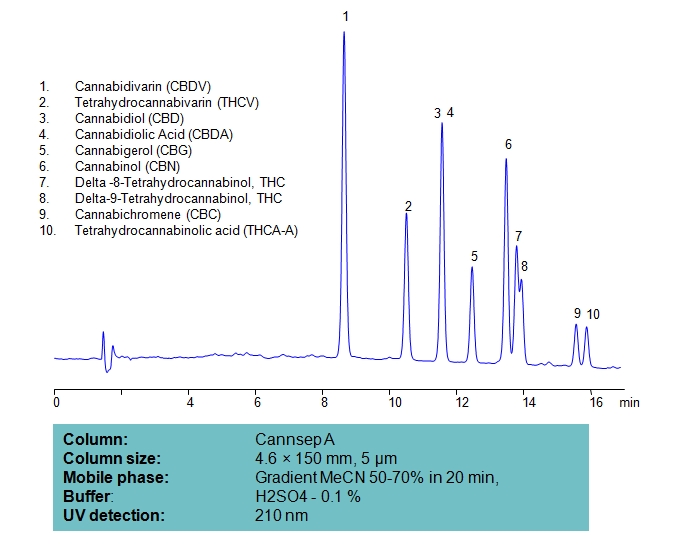
HPLC Separation of Ten Cannabinoids on Cannsep A Column
January 13, 2020
| Column | Cannsep A, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN 50-70% , 20 min |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Hydrophilic, Ionizable, Supplements |
| Analyzing Compounds | Cannabidivarin (CBDV), Tetrahydrocannabivarin (THCV), Cannabidiol (CBD), Delta-9-Tetrahydrocannabinol (THC), Cannabigerol (CBG), Cannabichromene (CBC), Tetrahydrocannabinolic acid (THCA-A) |
Application Column
Cannsep A
The Cannsep family of columns is a specially developed line of reverse-phase columns designed to retain and separate most of the compounds responsible for cannabis's physiological properties. With three options available - Cannsep A, Cannsep B, and Cannsep C - customers can resolve all the various cannabinoids, each providing unique and significantly orthogonal selectivity.
Select optionsCannabidiolic acid (CBDA)
Cannabidivarin (CBDV)
Cannabigerol (CBG)
Cannabinol (CBN)
Delta-8-Tetrahydrocannabinol (8-THC)
Delta-9-Tetrahydrocannabinol (THC)
Tetrahydrocannabinol (THC)
Tetrahydrocannabinolic acid (THCA-A)
Tetrahydrocannabivarin (THCV)

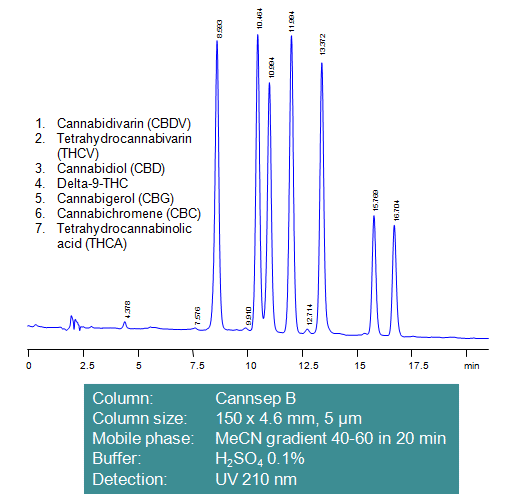
Fast Separation of Cannabinoids on Mixed-Mode HPLC Column Cannsep B
December 5, 2013
With legalization of marijuana in much of the United States, more and more attention is paid to developing quick, robust and reliable method for quantitation of cannabinoids in marijuana plants and related edible products. The main challenge lies in sample preparation and quantitative analysis of four major cannabinoids: cannabidiol, cannabinol, tetrahydrocannabinol and tetrahydrocannabinolic acid. All compounds are hydrophobic with only THCA (THC-A) and cannabidiolic acid (CBDA) being an acid. Hydrophobic interaction is the main interaction in the separation of cannabinoids on reverse phase columns. On our mixed mode columns acidic molecules retained by ion-exchange mechanism in addition to hydrophobic. Method can be used for analysis and prep separation of cannabinoids in marijuana plants, seeds and other cannabis-based products. Extraction of cannabinoids is the main procedure in sample preparation. Extraction can be done with organic solvents like chloroform, methanol, ethanol, acetonitrile. Cannabinoids can be monitored by UV and LC/MS. Mixed-mode columns containing polar embedded groups provide different selectivity than regular reversed-phase columns.
| Column | Cannsep B, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN 40-60% , 20 min |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Hydrophilic, Ionizable, Supplements |
| Analyzing Compounds | Cannabidivarin (CBDV), Tetrahydrocannabivarin (THCV), Cannabidiol (CBD), Delta-9-Tetrahydrocannabinol (THC), Cannabigerol (CBG), Cannabichromene (CBC), Tetrahydrocannabinolic acid (THCA-A) |
Application Column
Cannsep B
The Cannsep family of columns is a specially developed line of reverse-phase columns designed to retain and separate most of the compounds responsible for cannabis's physiological properties. With three options available - Cannsep A, Cannsep B, and Cannsep C - customers can resolve all the various cannabinoids, each providing unique and significantly orthogonal selectivity.
Select optionsCannabidiol (CBD)
Cannabidivarin (CBDV)
Cannabigerol (CBG)
Cannabinol (CBN)
Tetrahydrocannabinol (THC)
Tetrahydrocannabinolic acid (THCA-A)