| CAS Number | 504-29-0 |
|---|---|
| Molecular Formula | C5H6N2 |
| Molecular Weight | 94.117 |
| InChI Key | ICSNLGPSRYBMBD-UHFFFAOYSA-N |
| LogP | 0.48 |
| Synonyms |
|
Applications:
HPLC Separation of Aminopyridines in Non-Aqueous Mobile Phase
July 10, 2013
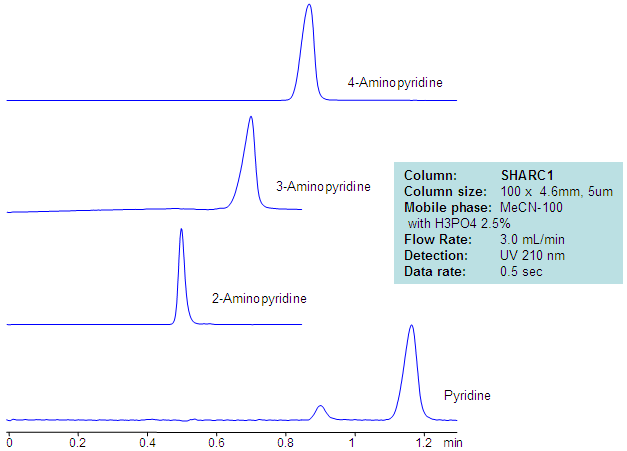
| Column | Sharc 1, 4.6×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN – 97.5% |
| Buffer | H3PO4 – 2.5% |
| Flow Rate | 3.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Muscle strengthener, Hydrophilic, Ionizable, Vertebrate pesticide |
| Analyzing Compounds | 2-Aminopyridine, 3-Aminopyridine, 4-Aminopyridine, Pyridine |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select options3-Aminopyridine
4-Aminopyridine
Pyridine

HPLC Separation of Aminopyridines Isomers in Hydrogen-Bonding mode on a SHARC 1 HPLC Column
July 3, 2012
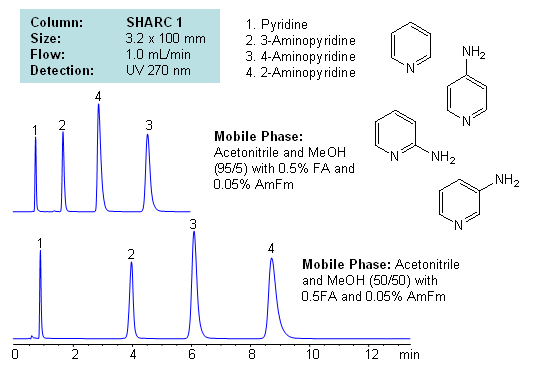
Application Notes: Pyridines and aminopyridines are hydrophilic basic compounds. Traditionally these compounds have been separated and analyzed by GC and HPLC. In the case of HPLC, reversed-phase chromatography with ion-pairing reagent is used along with alternative modes like HILIC for separation. Mixed-mode chromatography can also be used to successfully separate isomers of substituted pyridines. However, we developed a new mode of separation for these compounds with hydrogen bonding. Isomers of aminopyridine are separated based on hydrogen bonding interaction between analyte and stationary phase. Mobile phase utilizes combination of acetonitrile and methanol with additives. Retention time and selectivity are sensitive to variations of mobile phase. The order of elution changes depending on the amount of acetonitrile, methanol, formic acid and ammonium formate. This method and approach is compatible with LC/MS and prep chromatography and can be used for separation of other pyridine based compounds and pyridine based isomers.
Application Columns: SHARC 1, 3.2×100 mm, 5 um, 100A, To learn more about SHARC 1 columns click here. To order this column click here. To see more chromatographic separations check our web site.
Application Compounds: pyridine, 2-aminopyridine, 3-aminopyridine, 4-aminopyridine
Detection Technique: UV, LC/MS
| Column | Sharc 1, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH |
| Buffer | Fa, AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Muscle strengthener, Hydrophilic, Ionizable, Vertebrate pesticide |
| Analyzing Compounds | 2-Aminopyridine, 3-Aminopyridine, 4-Aminopyridine, Pyridine |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select options3-Aminopyridine
4-Aminopyridine
Pyridine
UV Detection

Comparison of Obelisc R to C18 columns for the Separation of Aminopyridine Isomers
March 3, 2010
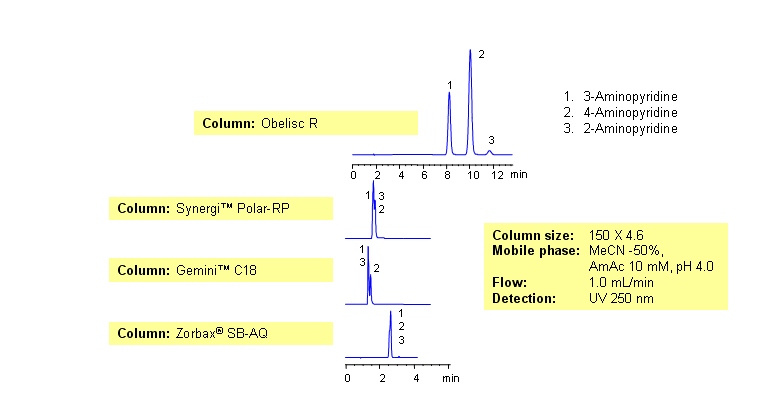
Three isomers of aminopyridine separate well by reversed-phase cation-exchange mixed-mode chromatography on an Obelisc R HPLC column. The presence of additional mechanism of interaction provides great selectivity for separation of closely related compounds. Elution of compounds can be monitored by UV, Evaporative Light-Scattering Detector (ELSD), Corona (CAD) or LC/MS. This method was validated at a pharmaceutical company. This HPLC method can be adopted as general approach for analysis of aminopyridine and other isomeric compounds.
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select options3-Aminopyridine
4-Aminopyridine
UV Detection