| CAS Number | 504-24-5 |
|---|---|
| Molecular Formula | C5H6N2 |
| Molecular Weight | 94.117 |
| InChI Key | NUKYPUAOHBNCPY-UHFFFAOYSA-N |
| LogP | 0.32 |
| Synonyms |
|
Applications:
HPLC Separation of Vitamin C, Vitamin Group B, and Related Impurities
August 22, 2008
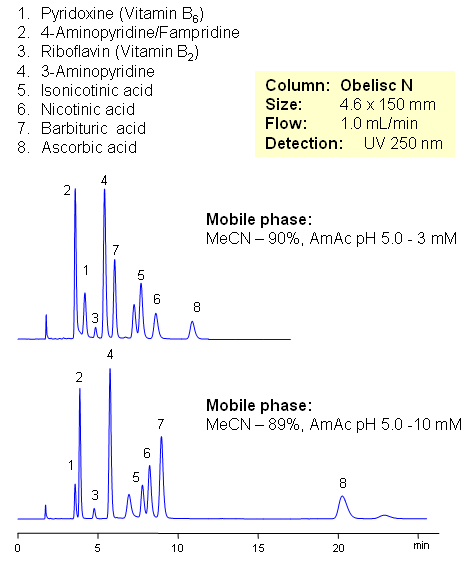
Vitamin C (ascorbic acid) and Vitamins Group B are separated on Obelisc N mixed-mode column. Method can be used in quantitation and determination of polar vitamins in various formulations and dietary supplements. HPLC method can be based on UV, Evaporative Light Scattering Detection (ESLD), RI or MS detection. Effect of sample matrix can be eliminated by changing mobile phase conditions. Buffer concentration, buffer pH and amount of ACN will affect every vitamin differently due to difference in polar and ionic properties.
| Column | Obelisc N , 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc pH 5.0 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Vitamin B₆, Hydrophobic, Ionizable |
| Analyzing Compounds | Pyridoxine, Ascorbic acid, Niacinamide, Pantothenic acid, Caffeine, Riboflavin, Barbituric Acid, 3- Aminopyrine |
Application Column
Obelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select options3-Aminopyridine
4-Aminopyridine/Fampridine
Ascorbic Acid
Barbituric Acid
Isonicotinic Acid
Nicotinic Acid/Niacin (3-pyridinecarboxylic acid)
Vitamin B2 (Riboflavin)
Vitamin B6 (Pyridoxine)