| CAS Number | 65-49-6 |
|---|---|
| Molecular Formula | C7H7NO3 |
| Molecular Weight | 153.137 |
| InChI Key | WUBBRNOQWQTFEX-UHFFFAOYSA-N |
| LogP | 0.89 |
| Synonyms |
|
Applications:
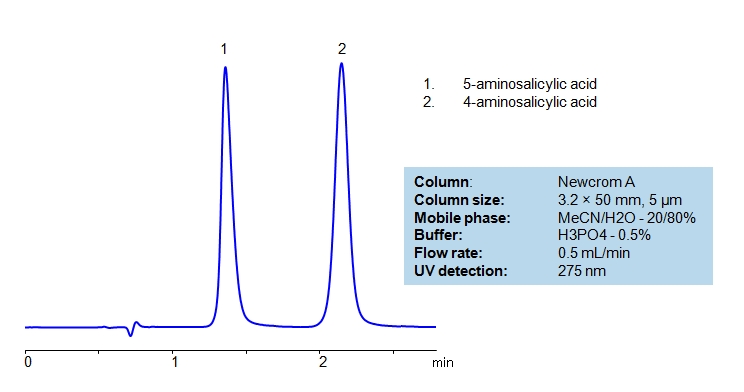
HPLC Separation of Isomers of Amino Salicylic Acid
November 21, 2010
| Column | Newcrom A, 4.6×50 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 20/80% |
| Buffer | H3PO4 – 0.5% |
| Flow Rate | 0.5 ml/min |
| Detection | UV 275 nm |
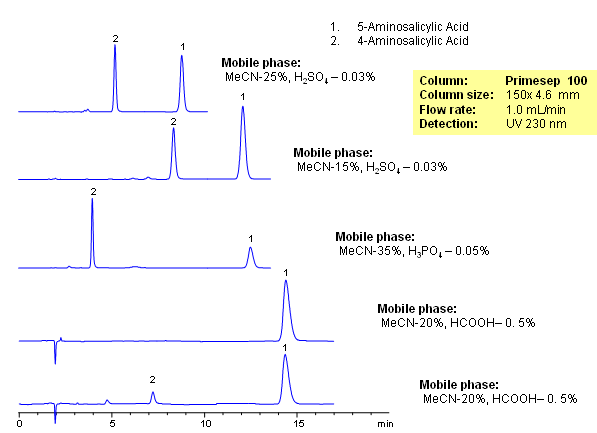
4-Aminosalicylic acid (PAS) is an antibiotic used in treatment of tuberculosis. It is a polar amino acid with limited retention on traditional C18 (reversed-phase) columns. Other isomers of aminosalicylic acid exist, but the main impurity in PAS is 5-Aminosalicylic acid, which also serves as anti-inflammatory drug. Both compounds are isomers with similar empirical structure and properties. These two isomers were separated on a Primesep 100 column with UV, ELSD and LC/MS compatible mobile phase. Method can be used a generic approach for separation of isomers of basic and zwitter ionic compounds. Isomers are retained and separated based on reversed-phase and cation-exchange properties. Retention time is controlled by the amount of acetonitrile, buffer concentration and buffer pH. Buffer pH is affecting ionization of these two compounds and thus serves as a powerful tool to adjust selectivity of separation.
| Column | Newcrom A, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 50/50% |
| Buffer | AmFm pH 3.0- 40 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 256 nm, MS-compatible mobile phase |
| Class of Compounds | Acids |
| Analyzing Compounds | 5-aminosalicylic acid, 4-aminosalicylic acid |
Application Column
Newcrom A
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsPrimesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options5-Aminosalicylic Acid (Mesalamine)
Mesalamine hydrochloride