| CAS Number | 50-48-6 |
|---|---|
| Molecular Formula | C20H23N |
| Molecular Weight | 277.412 |
| InChI Key | KRMDCWKBEZIMAB-UHFFFAOYSA-N |
| LogP | 4.92 |
| Synonyms |
|
Applications:
Generic Screening Method for Complex Mixtures
October 15, 2015
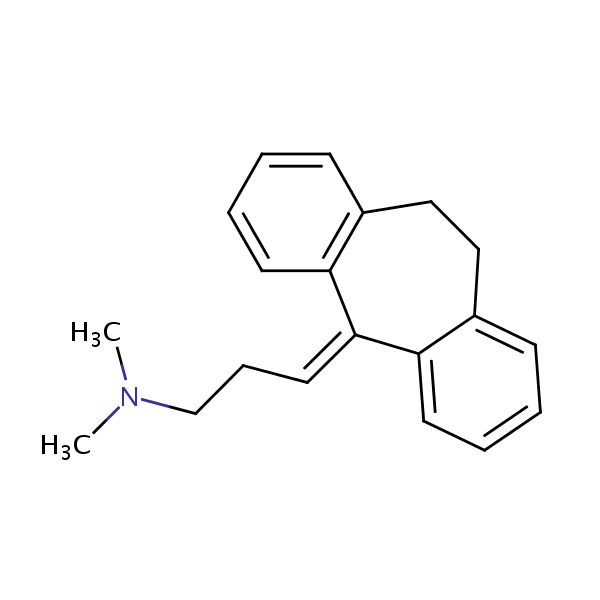
| Column | Primesep 200, 4.6*150 mm 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 215 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Uracil, Epinephrine, DOPA, 2,6-Lutidine, Benzylamine, Hydroxytrypthophan, Homovanillic acid, Phenol, Tryptophan , 2,3-DHBA, Benzoic acid, Methylparaben, Ethylparaben, Toluene, Amitriptyline |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options2,6-Lutidine
Amitriptyline
Benzoic Acid
Benzylamine
DOPA (3,4-dihydroxy-L-phenylalanine)
Epinephrine
Ethylparaben
Homovanillic Acid
Hydroxytryptophan
Methylparaben
Phenol
Toluene
Tryptophan
Uracil

USP Methods for the Analysis of Amitriptyline using a Legacy L1 Column
June 21, 2012
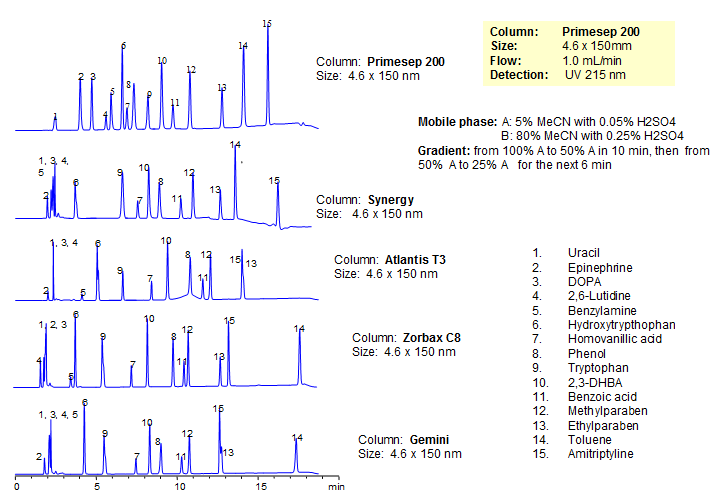
Application Notes: Amitriptyline is a tricyclic antidepressant. While it is a older drug, it is still just as effective as newer SSRI’s. According to the USP methods, amitriptyline hydrochloride conatin no less than 99% and no more than 100.5% of amitriptyline. The USP HPLC method for the separation of amitriptyline was developed on Legacy L1 column according to the US Pharmacopeia methodology. L1 classification is assigned to reversed-phase HPLC column containing C18 ligand. Support for the material is spherical silica gel with particles size 3-10 um and pore size of 100-120A. Resolution between critical pairs corresponds to rules and specifications of USP.
Application Columns: Legacy L1 C18 HPLC column
Application compounds: Amitriptyline
Mobile phase: MeCN/92mM monobasic sodium
Detection technique: UV
Reference: USP35: NF30
| Column | Legacy L1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/92 mM NaH2PO4 pH 2.5 (42/58) |
| Buffer | NaH2PO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Nerve pain medication and antidepressant, Hydrophobic, Ionizable |
| Analyzing Compounds | Amitriptyline |
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select options
HPLC Separation of 8 Generic Compounds on Primesep Columns
March 27, 2011
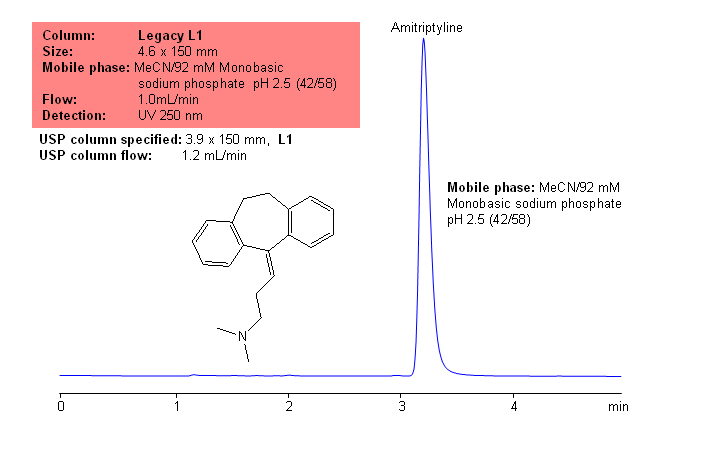
Mixed-mode HPLC columns allow to analyze compounds with drastically different properties in one run. Acidic, basic, and neutral compounds can be separated in one run using either isocratic or gradient conditions. In this application, neutral hydrophilic (uracil, phenol and hydroquinone), neutral hydrophobic (toluene), hydrophilic acidic (benzoic acid), hydrophilic basic (lutidine) and hydrophobic basic (amitriptyline) are separated using gradient of ACN. Neutral compounds are retained by reversed-phase mechanism, hydrophilic acidic compound become more hydrophobic at lower pH and retain by reversed-phase mechanism too. Basic compounds are retained by cation exchange mechanism, and hydrophobic basic compounds are retained by reversed-phase and cation-exchange mechanisms. All compounds are resolved within 17 minutes on a short column. Method can be applied to various polar and hydrophobic compounds, which can be separated on one column and in one run. Mixed-mode columns can operate in single or combination of several modes: reversed-phase, ion-exchange, ion-exclusion and HILIC. This mixed-mode HPLC column can be used as a general column for separation of wide range of compounds.
| Column | Primesep 200, Primesep 100 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm pH3.0 |
| Flow Rate | 0.5 ml/min, 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Uracil, Hydroquinone, Phenol, Benzoic Acid, Benzylamine, Lutidine, Toluene, Amitriptyline |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsBenzoic Acid
Benzylamine
Hydroquinone
Lutidine
Phenol
Toluene
Uracil

HPLC Separation of Acidic, Basic, and Neutral Compounds
November 21, 2010

Primesep 100 and Primesep 200 columns can be used as a universal column for analysis of wide range of compounds. These mixed-mode reversed-phase ion-exchange HPLC columns can provide a valuable alternative to traditional reversed-phase column. Amines, amino acids, quaternary amines, and various zwitter-ions can be analyzed along with hydrophobic compounds and organic and inorganic counter-ions. In this application, 8 compounds with different hydrophobic, hydrophilic, basic and acidic properties are separated based on their properties. Primesep 100 column is a mixed-mode HPLC column with a C12 carbon chain and carboxylic acid on the surface with pKa of 1. Primesep 200 column is a mixed-mode HPLC column with a C12 carbon chain and carboxylic acid on the surface with pKa of 2. These columns can be used with 100% organic (ACN) and 100% aqueous mobile phases. This HPLC method can be adopted as a generic and robust approach for analysis of acidic, basic and neutral compounds within the same run.
| Column | Primesep 200, 3.0×50 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm pH3.0 |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Uracil, Hydroquinone, Phenol, Benzoic Acid, Benzylamine, Lutidine, Toluene, Amitriptyline |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsBenzoic Acid
Benzylamine
Hydroquinone
Lutidine
Phenol
Toluene
Uracil

HPLC Analysis of Drugs in Saliva
August 22, 2010

The presence of three drugs, dextromethorphan, amitriptyline and trimipramine in saliva were analyzed by mixed-mode chromatography on a Primesep D reversed-phase anion-exchange column. No sample preparation is required as proteins from saliva are not retained at these conditions and elute in the void of the column due to cation-exclusion effect. Both column and proteins are positively charged at current conditions. Mobile phase consists of ACN-water-formic acid and is compatible with LC/MS detection technique. This general HPLC method can be used for analysis of hydrophobic basic drugs in biofluids.
Application Column
Primesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAmitriptyline
Dextromethorphan

Peak Symmetry Study with Amitryptiline
September 18, 2005
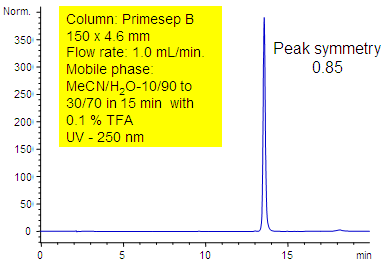
Primesep B has been applied to the analysis of amitriptyline. Amitriptyline is a tertiary amine commonly used a probe in reversed-phase columns to test silanol activity. On C18 phases amitriptyline often tails unless mobile phase modifiers such as triethylamine or high ionic strength buffers are used. Primesep B has an embedded cation which shields the silica by ionic repulsion with the amitriptyline cation. Excellent peak shape results with a mass spec compatible mobile phase of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA).
Application Column
Primesep B
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options