| CAS Number | 26787-78-0 |
|---|---|
| Molecular Formula | C16H19N3O5S |
| Molecular Weight | 365.400 |
| InChI Key | LSQZJLSUYDQPKJ-NJBDSQKTSA-N |
| LogP | 0.87 |
| Synonyms |
|
Applications:
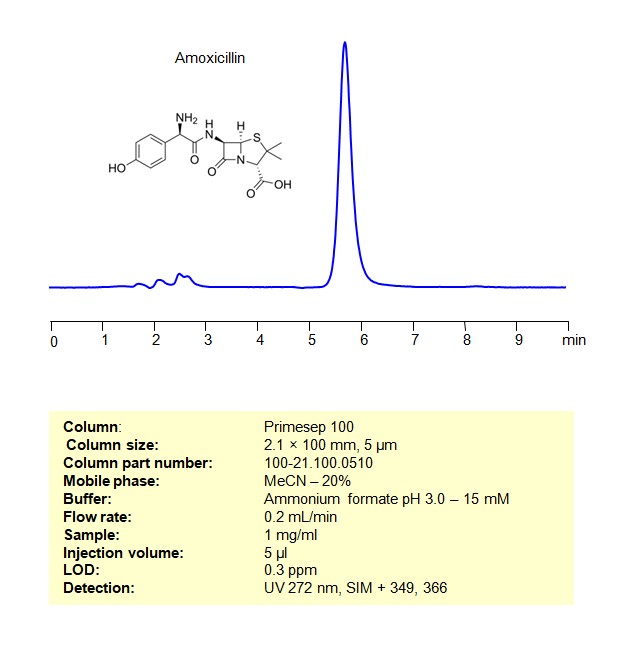
HPLC MS Method for Analysis of Amoxicillin on Primesep 100 Column
February 26, 2024
HPLC MS Method for Analysis of Procainamide on Primesep 100 Column
Separation type: Liquid Chromatography Mixed-mode SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Amoxicillin
Amoxicillin is a widely used antibiotic belonging to the penicillin class. It is used to treat a variety of bacterial infections, including respiratory tract infections, ear infections, skin infections, urinary tract infections, and certain types of bacterial endocarditis.
- Mechanism of Action: Amoxicillin works by inhibiting the synthesis of bacterial cell walls. It does this by interfering with the formation of peptidoglycan, an essential component of the bacterial cell wall.
- Spectrum of Activity: Amoxicillin is effective against a broad spectrum of bacteria, including both Gram-positive and Gram-negative bacteria. However, its effectiveness may be limited against bacteria that produce beta-lactamase enzymes.
- Administration: It is commonly available in oral formulations, such as capsules, tablets, and oral suspensions. There are also intravenous formulations for more severe infections.
- Common Uses:
- Respiratory Tract Infections: Amoxicillin is often prescribed for conditions like bronchitis and pneumonia.
- Ear Infections: It is used to treat middle ear infections.
- Skin Infections: Amoxicillin can be effective against various skin infections.
- Urinary Tract Infections (UTIs): It is sometimes prescribed for uncomplicated UTIs.
Amoxicillin can be retained and analyzed using a Primesep 100 mixed-mode stationary phase column. The analysis employs an isocratic method with a simple mobile phase comprising water, acetonitrile (MeCN), and ammonium formate as a buffer. This method allows for detection using UV at 272 nm
| Column | Primesep 100, 2.1 x 100 mm, 5 µm, 100 A |
| Mobile Phase | MeCN 20% |
| Buffer | AmFm pH 3.0 – 15 mM |
| Flow Rate | 0.2 ml/min |
| Detection | UV 272nm, SIM + 349, 366 |
| Samples | 1 mg/ml |
| Injection volume | 5 µl |
| LOD* | 0.3 ppm |
| Class of Compounds | Drug |
| Analyzing Compounds | Amoxicillin |
Application Column
Primesep 100
Column Diameter: 2.1 mm
Column Length: 100 mm
Particle Size: 5 µm
Pore Size: 100 A
LC MS Detection

HPLC Separation of β-Lactam Antibiotics on Primesep 100 Column
March 18, 2021
Separation type: Liquid Chromatography Mixed-mode
View on hplc.cloud
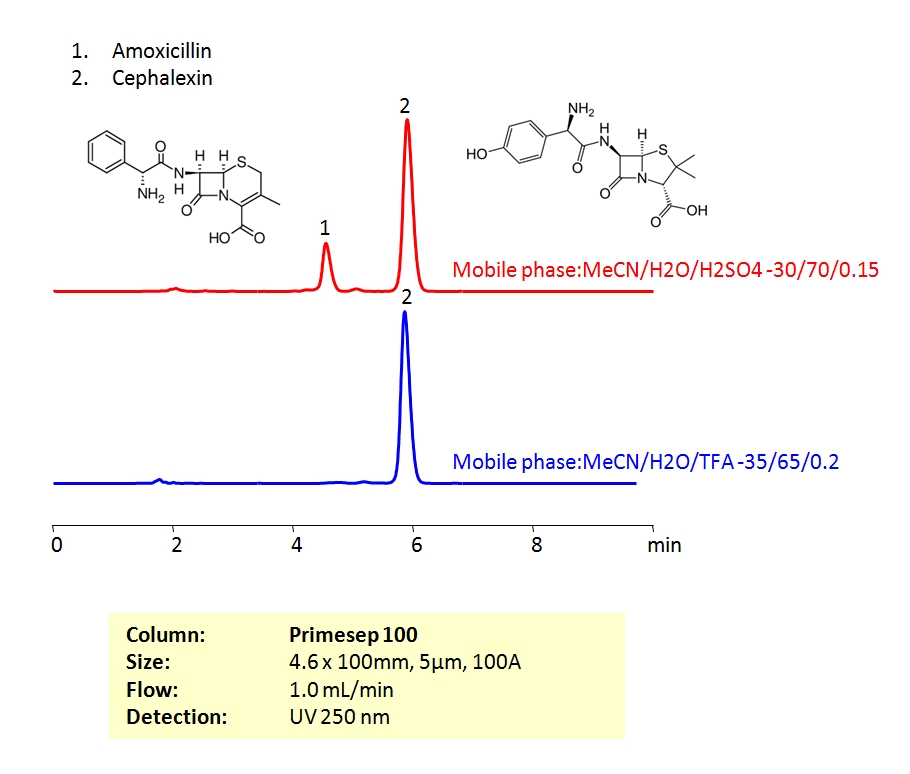
High Performance Liquid Chromatography (HPLC) Method for Analysis of Amoxicillin and Cephalexin.
Both amoxicillin and cephalexin are beta-lactam antibiotics. Beta-lactam antibiotics contain a beta-lactam ring in their molecular structure. As a group, these drugs are active against many gram-positive, gram-negative and anaerobic organisms. Both compounds can be retained and baseline separated with an isocratic method in 30/70 Acetonitrile (ACN) and water mobile phase with a Sulfuric acid (H2SO4) buffer. UV detection at 250 nm.
| Column | Primesep 100, 4.6×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | TFA, H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Antibiotics, Hydrophobic, Ionizable |
| Analyzing Compounds | Cephalexin, Amoxicillin |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAmoxicillin hydrate (1:3)
Cephalexin

HPLC Separation of Albuterol and Amoxicillin
March 27, 2015
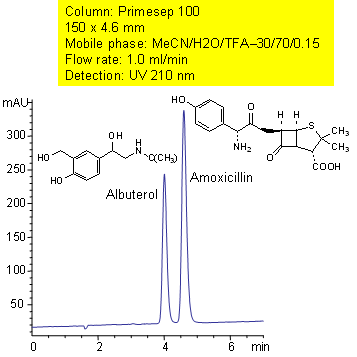
Primesep 100 separates albuterol and amoxicillin with baseline resolution by a combination of cation-exchange, hydrophobic, and polar interactions. The mass spec (LC/MS) compatible separation uses a mobile phase mixture of water, acetonitrile (MeCN, ACN) and trifluoroacetic acid (TFA) with UV detection at 210 nm.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | TFA – 0.15% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Antibiotics, Hydrophobic, Ionizable |
| Analyzing Compounds | Albuterol, Amoxicillin |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAmoxicillin

USP Method for the Analysis of Amoxicillin using the Legacy L1 Column
June 21, 2012
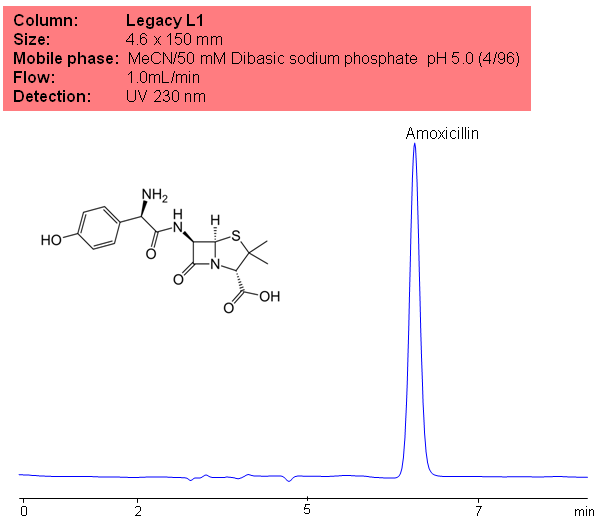
Application Notes: Amoxicillin is one of the most commonly prescribed antibiotics. It is often used for treating strep throat. According to USP methods, amoxicillin should not contain less than 900ug and no more than 1050ug of amoxicillin per mg. The USP HPLC method for the separation of amitriptyline was developed on Legacy L1 column according to the US Pharmacopeia methodology. L1 classification is assigned to reversed-phase HPLC column containing C18 ligand. Support for the material is spherical silica gel with particles size 3-10 um and pore size of 100-120A. Resolution between critical pairs corresponds to rules and specifications of UPS.
Application Columns: Legacy L1 C18 HPLC column
Application compounds: Amoxicillin
Mobile phase: MeCN/50 mM dibasic sodium phosphate
Detection technique: UV
Source: USP35: NF30
| Column | Legacy L1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/50 mM NaH2PO4 pH 5.0 (4/96) |
| Buffer | NaH2PO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 230 nm |
| Class of Compounds |
Drug, Antibiotics, Hydrophobic, Ionizable |
| Analyzing Compounds | Amoxicillin |
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select options