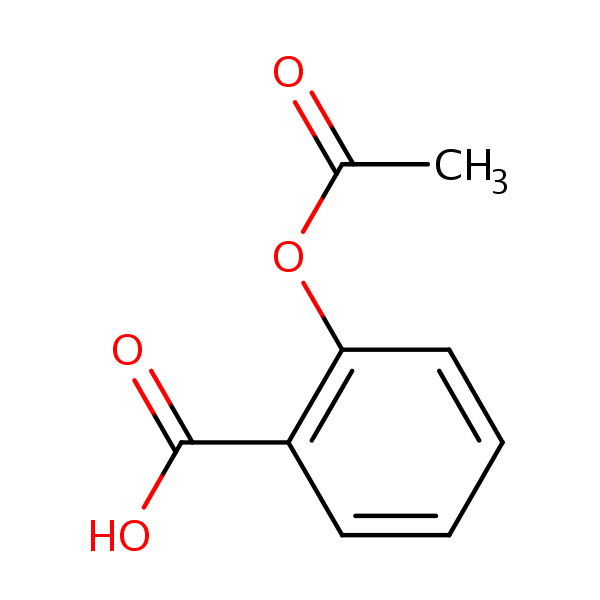
| CAS Number | 50-78-2 |
|---|---|
| Molecular Formula | C9H8O4 |
| Molecular Weight | 180.159 |
| InChI Key | BSYNRYMUTXBXSQ-UHFFFAOYSA-N |
| LogP | 1.19 |
| Synonyms |
|
Applications:
USP Methods for the Analysis of an Analgesic Mixture Using the Legacy L1 Column
June 21, 2012
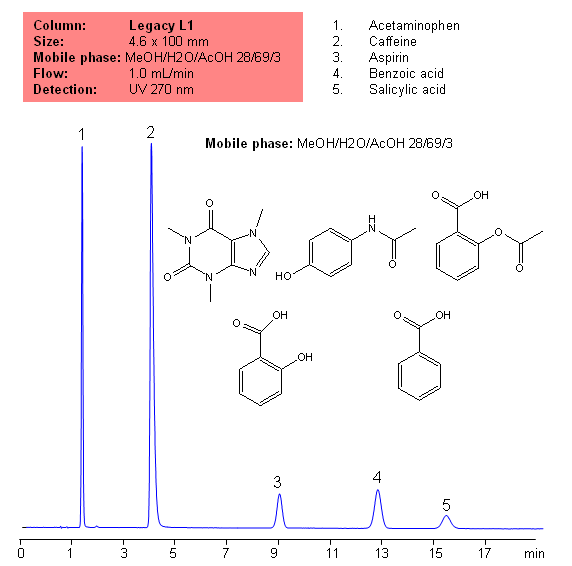
Application Notes: Acetametaphin, aspirin, and caffeine tablets contain not less than 90 percent and not more than 110 percent of the labeled amounts if acetametaphin, asprin, and caffeine according the USP methods. USP HPLC method for separation of acetaminophen, aspirin and caffeine was developed on Legacy L1 column according to US Pharmacopeia methodology. L1 classification is assigned to reversed-phase HPLC column contains C18 ligands. Support for the material is a spherical silica gel with particles size 3-10 um and pore size of 100-120A. Resolution between critical pairs corresponds to rules and specifications of USP.
Application Columns: Legacy L1 C18 HPLC column
Application compounds: Acetaminophen, Aspirin, Caffeine, benzoic acid, and salicylic acid
Mobile phase: MeOH/H2O/AcOH 28/69/3
Detection technique: UV
Reference: USP30: NF35
| Column | Legacy L1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeOH/H2O/AcOH 28/69/3 |
| Buffer | AcOH |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophobic, Ionizable |
| Analyzing Compounds | Acetaminophen, Caffeine, Aspirin, Benzoic acid, Salicylic acid |
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select optionsAspirin
Benzoic Acid
Caffeine

USP Methods for the Analysis of Aspirin (Acetylsalicylic acid (ASA)) Using Legacy L1 Column
June 21, 2012
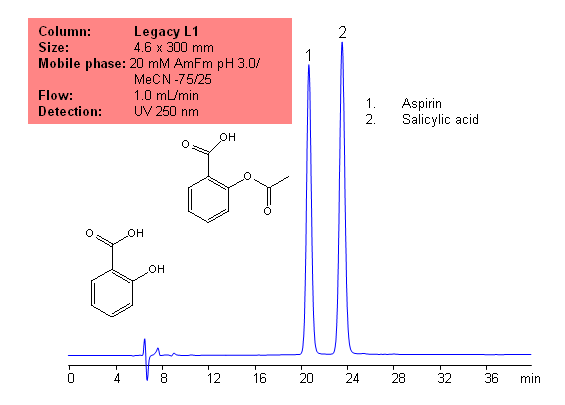
Application Notes: Aspirin is one of the oldest analgesics. While it is one of the oldests analgesics, it is still widely used today, and is still one of the most common drugs. According to the USP methods, aspirin contains not less than 99.5% and no more than 100.5 percent of aspirin calculate on a dried basis. The USP HPLC method for the separation of aspirin was developed on Legacy L1 column according to the US Pharmacopeia methodology. L1 classification is assigned to reversed-phase HPLC column containing C18 ligand. Support for the material is spherical silica gel with particles size 3-10 um and pore size of 100-120A. Resolution between critical pairs corresponds to rules and specifications of USP.
Application Columns: Legacy L1 C18 HPLC column
Application compounds: Aspirin and salicylic acid
Mobile phase: 20 mM AmFm pH 3.0/MeCN- 75/25
Detection technique: UV
Reference: USP35: NF30
| Column | Legacy L1, 4.6×300 mm, 5 µm, 100A |
| Mobile Phase | MeCN – 25% |
| Buffer | AmFm pH 3.0 20 mM – 75% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophobic, Ionizable |
| Analyzing Compounds | Aspirin (Acetylsalicylic acid (ASA)) |
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select optionsAspirin

HPLC Separation of Components of Excedrin (Benzoic acid, Acetaminophen, Caffeine, Aspirin)
July 16, 2009
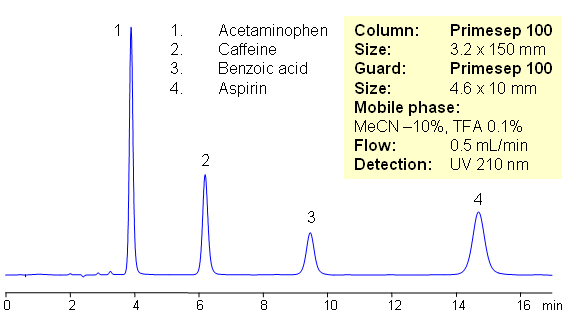
Excedrin is over-the-counter pain reliever containing acetaminophen, caffeine and aspirin as active ingredients of this drug composition. Acetaminophen (paracetamol) is used as analgesic and pain reliever. It is a neutral compound with low hydrophobicity. Aspirin or acetylsalicylic acid is used as analgesic and anti-inflammatory component of many OTC compositions. It is weakly acidic and slightly hydrophobic compound. Caffeine is xanthine alkaloid which is psychoactive stimulant drug. All four compounds are separated on mixed-mode Primesep 100 HPLC column with acetonitrile/water/TFA mobile phase. In this HPLC application compounds are retained by reversed phase mechanism. This HPLC method is short and robust.
| Column | Primesep 100, 3.2×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | TFA |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Benzoic acid, Acetaminophen, Caffeine, Aspirin |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAspirin
Benzoic Acid
Caffeine
UV Detection