| CAS Number | 100-47-0 |
|---|---|
| Molecular Formula | C7H5N |
| Molecular Weight | 103.125 |
| InChI Key | JFDZBHWFFUWGJE-UHFFFAOYSA-N |
| LogP | 1.56 |
| Synonyms |
|
Applications:
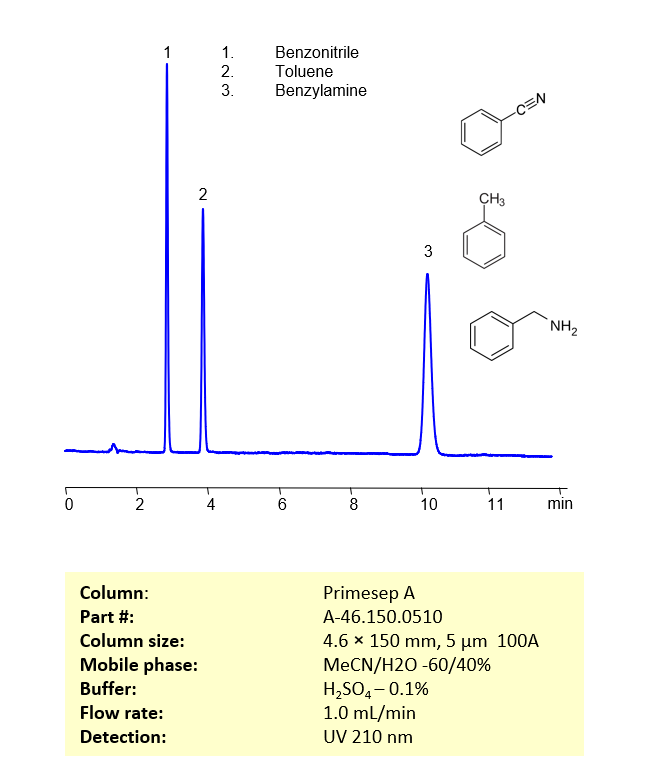
HPLC Method for Separation of Benzonitrile, Toluene and Benzylamine on Primesep A Column
May 31, 2023
HPLC Method for Analysis of Benzonitrile, Toluene, Benzylamine on Primesep A by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
- Benzonitrile: Benzonitrile (C₆H₅CN) is an aromatic organic compound. It is a colorless liquid with an almond-like odor. Benzonitrile is used as a solvent and a chemical intermediate in the production of a variety of compounds, including pharmaceuticals, dyes, and perfumes.
- Toluene: Toluene (C₇H₈) is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a CH₃ group attached to a phenyl group. It is an important organic solvent and is also used to produce benzene, and a number of consumer products including certain types of glue, paint thinners, and nail polish removers.
- Benzylamine: Benzylamine (C₆H₅CH₂NH₂) is a primary amine compound containing a benzyl group attached to the amino group. It is a colorless liquid with a faint almond-like odor. It is used as a chemical intermediate in the synthesis of a variety of substances, including pharmaceuticals, dyes, and polymers.
These chemicals are widely used in organic synthesis. Please handle these substances with care, as they can have hazardous effects. Always refer to the safety data sheets for each chemical and adhere to recommended safety procedures.
These three compounds be separate and analyzed on a reverse-phase Primesep A, 4.6 x 150 mm, 5 µm, 100 A column with a mobile phase consisting of water, Acetonitrile (MeCN) and Sulfuric acid as a buffer modifier. This analysis method can be UV detected at 210 nm.
LOD was determined for this combination of instrument, method, and analyte, and it can vary from one laboratory to another even when the same general type of analysis is being performed.
High Performance Liquid Chromatography (HPLC) Method for Analysis of Benzonitrile, Toluene, Benzylamine
Condition
| Column | Primesep A, 4.6 x 150 mm, 5 µm, 100 A |
| Mobile Phase | MeCN/H2O -60/40% |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV 210 nm |
| Peaks Retention Time | 2.9, 4.0, 10.2 min |
| Samples concentration (Benzonitrile, Toluene, Benzylamine) | 0.04, 0.05, 0.07 mg/ml |
| Injection volume | 3 µl |
| Sample diluent | MeCN/H2O -60/40% |
| LOD (Benzonitrile, Toluene, Benzylamine) | 27, 65, 103 ppb |
Description
| Class of Compounds | Benzene, Aromatics |
| Analyzing Compounds | Benzonitrile, Toluene, Benzylamine |
Application Column
Primesep A
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Benzylamine
Toluene

HPLC Separation of Methyl Paraben, Benzonitrile, Propyl Paraben, and Toluene on Mixed-Mode and Reverse Phase Columns
October 4, 2010
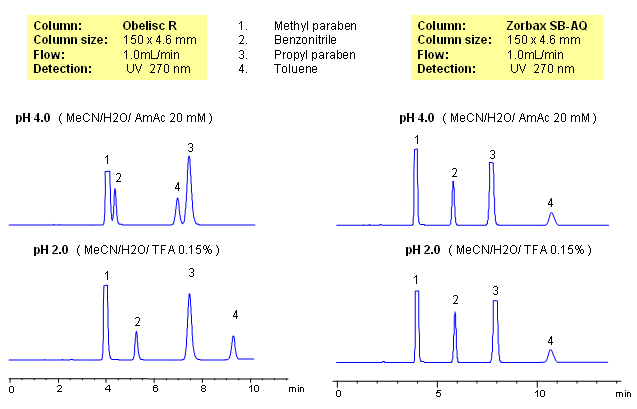
Parabens are common preservatives in pharmaceutical and cosmetic industries. They are esters of p-hydroxybenzoic acid. Method for separation of methyl paraben, propyl paraben, benzonitrile and toluene was developed on a Obelisc R column. All four compounds are neutral and are retained by reverse-phase mechanism. In case of reversed-phase stationary phase, no effect of pH is observed. Retention time for all four compounds changes on an Obelisc R column when pH is changed. pH of the mobile phase affects ionization state of stationary phase. Obelisc R column has C12 carbon chain and carboxylic acid with pKa of 4. At lower pH (pH 2, TFA), carboxylic acid of stationary phase is not ionized and thus adds hydrophobicity to stationary phase. Obelisc R column can be used for analysis of basic, acidic and neutral compounds with suitable detection techniques – UV, ELSD, CAD, LC/MS.
| Column | Obelisc R, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc, TFA |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Preservatives, Neutral |
| Analyzing Compounds | Methylparaben, Benzonitrile, Propyl paraben, Toluene |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsMethylparaben
Propylparaben
Toluene

Acid Effect on Retention of Acidic Analytes
July 8, 2010
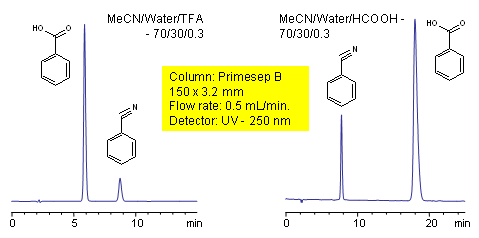
Benzonitrile

Effect of mobile phase composition on retention of 3 compounds on Obelisc R
March 3, 2010
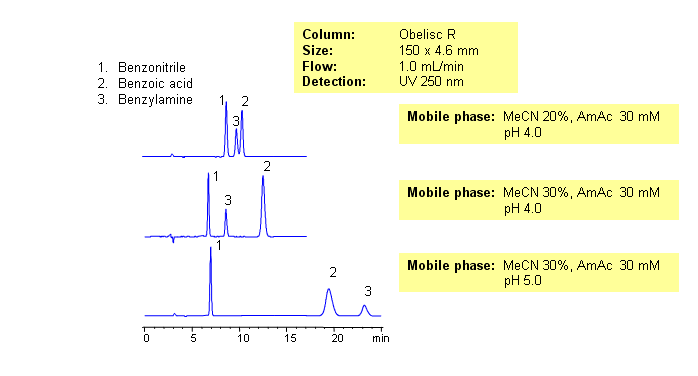
This application shows the effect of mobile phase composition on retention of acidic, basic and neutral compound. Retention of basic and acidic compounds is controlled by buffer pH and concentration, while retention of neutral compound is controlled by the amount of acetonitrile. Buffer pH changes not only ionization state of ionizable basic and acidic compounds, but ionization state of trimodal stationary phase Obelisc R. Available detection techniques include UV, ELSD, LC/MS, and Corona.
| Column | Obelisc R, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc |
| Flow Rate | 1.0 ml/min |
| Detection | 250 |
| Class of Compounds |
Acid, Hydrophilic, Neutral, BasicIonizable |
| Analyzing Compounds | Benzonitrile, Benzoic Acid, Benzylamine |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsBenzonitrile
Benzylamine

HPLC Separation of Dequalinium and Benzonitrile on Different Columns
August 22, 2008
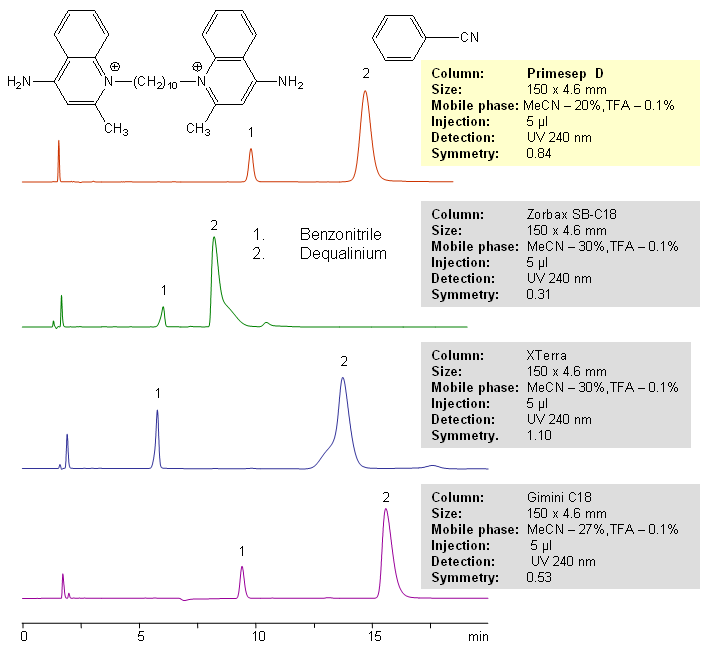
Dequalinium, or Dequadin, is an antiseptic and disinfectant. It consists of two quaternary amines connected by C10 hydrophobic chain. It’s a strongly basic compound that is hard to analyze on reverse phase column and obtain good peak shape. Compound shows a significant tailing (symmetry below 0.5 and USP tailing factor of over 2) due to interaction of quaternary groups with residual silanols. In this HPLC application dequalinium is analyzed on Primesep D mixed-mode column with perfect symmetry and high efficiency. Primesep D column has hydrophobic chain and a basic group close to the surface of silica gel. This group provides shielding effect and prevents interaction between acidic silanol groups and quaternary amines of dequalinium. Method can be used for analysis and quantitation of hydrophobic amines when peak shape is not satisfactory on traditional HPLC columns. Relative comparison of several modern LC columns (Zorbax from Agilent, XTerra from Waters and Gemini from Phenomenex) shows that Primesep D mixed-mode column can be successfully used in determination of hydrophobic quaternary amines and diamines. If a compound is retained on C18 column with poor peak shape, switching to mixed-mode column can provide a valuable alternative. Benzonitrile is used to ensure high quality of packing and good peak shape for neutral analytes. Method uses trifluoroacetic acid as an additive to the mobile phase. Other acids and buffers can be used depending on the detection technique.
| Column | Primesep D, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | TFA |
| Flow Rate | |
| Detection | UV, 240 nm |
| Class of Compounds |
Quaternary amines |
| Analyzing Compounds | Dequalinium, Benzonitrile |
Application Column
Primesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDequalinium

HPLC Separation of Amino Acids, Bases, Acids, and Neutrals on Obelisc R
March 3, 2007
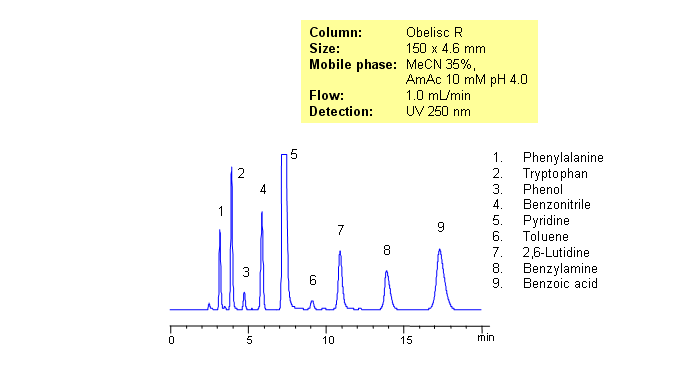
Separating basic, acidic and zwitterionic compounds in one run in reverse-phase HPLC can be very challenging. The methods might require the use of ion-pairing reagents and complex gradients that can make MS-compatibility difficult. Obelisc R column which has both positive and negative ion-pairs embedded in the stationary phase allows for fine tuning and separation of a wide range of compounds with different ionic properties. Acids, bases, amino acids and neutral compounds were separated isocratically in one run using a simple MS-compatible mobile phase of acetonitrile (ACN) and water with Ammonium Acetate (AmAc) buffer. Can also be UV detected at 250nm.
| Column | Obelisc R, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 35/65% |
| Buffer | AmAc 10 mM pH 4.0 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Bases, Neutral, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Amino acids |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsBenzoic Acid
Benzonitrile
Benzylamine
Phenol
Phenylalanine
Pyridine
Toluene
Tryptophan

Complex Mixture of Acids, Bases, Amino Acids, and Neutral Compounds
October 14, 2006
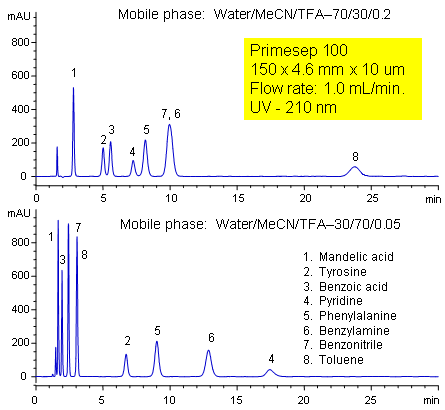
Primesep 100 separates a mixture of amino acids (tyrosine, phenylalanine), organic acids (benzoic acid, mandelic acid), amines (benzylamine, pyridine), and neutrals (benzonitrile, toluene) in one HPLC run by combining reversed-phase, cation-exchange, and polar interactions. The method is tunable and peak order can be changed significantly by adjusting acetonitrile and trifluoroacetic acid concentrations. The separation method uses a mobile phase mixture of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) and compatible with UV, mass spec (LC/MS) and evaporative light scattering (ELSD) detection.
| Column | Primesep 100, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | TFA – 0.2 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Tyrosine, phenylalanine, Benzoic acid, mandelic acid, Benzylamine, Pyridine, Benzonitrile, Toluene |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsBenzoic Acid
Benzonitrile
Benzylamine
Mandelic Acid
Organic Acids
Phenylalanine
Pyridine
Toluene
Tyrosine

Neutral, Base, and Acid on Primesep B Column
November 15, 2005
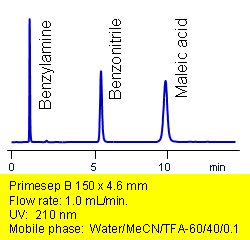
Primesep B separates acids, bases, and neutrals in one injection. Maleic acid, benzonitrile, and benzylamine are baseline resolved by a combination of reversed-phase, ion-exchange, and ion-exclusion mechanisms. Excellent peak shape results with a mass spec compatible mobile phase of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) with UV detection at 210 nm.
Application Column
Primesep B
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsBenzylamine
Maleic Acid

Neutral, Base, and Acid on Primesep A Column
November 14, 2005
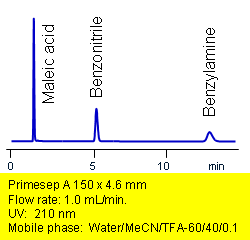
Primesep A separates acids, bases, and neutrals in one injection. Maleic acid, benzonitrile, and benzylamine are baseline resolved by a combination of reversed-phase, ion-exchange, and ion-exclusion mechanisms. Excellent peak shape results with a mass spec compatible mobile phase of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) with UV detection at 210 nm.
Application Column
Primesep A
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsBenzylamine
Maleic Acid

Neutral, Base, and Acid on Primesep 100 Column
November 14, 2005
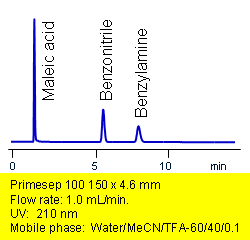
Primesep 100 retains and separates an acid, base, and neutral in one HPLC injection. Maleic acid, benzylamine, and benzonitrile are resolved by ion-exclusion, ion-exhange and reversed-phase modes. The separation uses a mobile phase of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) with UV detection at 210 nm.
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsBenzylamine
Maleic Acid

Neutral, Base, and Acid on Primesep 200 Column
November 14, 2005
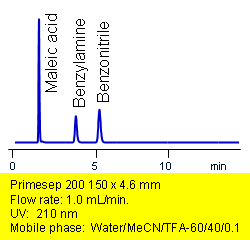
Primesep 200 separates acids, bases, and neutrals in one injection. Maleic acid, benzonitrile, and benzylamine are baseline resolved by a combination of reversed-phase, ion-exchange, and ion-exclusion mechanisms. Excellent peak shape results with a mass spec compatible mobile phase of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) with UV detection at 210 nm.
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsBenzylamine
Maleic Acid