| CAS Number | 58-08-2 |
|---|---|
| Molecular Formula | C8H10N4O2 |
| Molecular Weight | 194.195 |
| InChI Key | RYYVLZVUVIJVGH-UHFFFAOYSA-N |
| LogP | -0.0700 |
| Synonyms |
|
Applications:
UV-Vis Spectrum of Caffeine
July 11, 2025
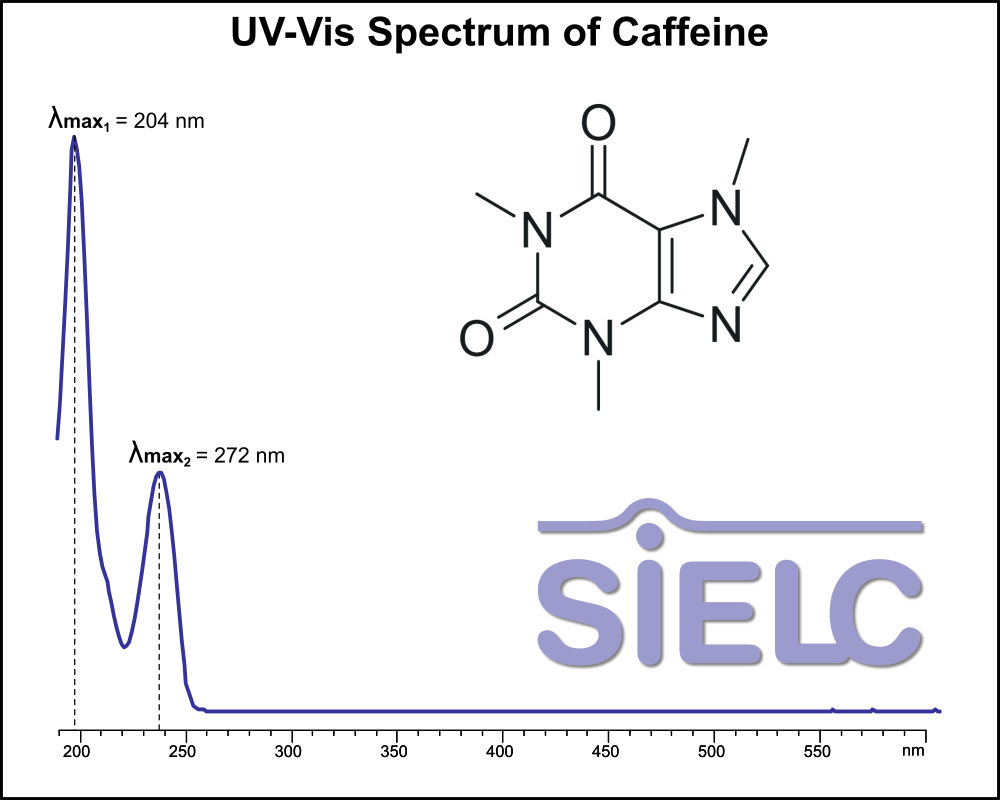
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.
For some compounds, the UV-Vis Spectrum is affected by the pH of the mobile phase. The spectra presented here are measured with an acidic mobile phase that has a pH of 3 or lower.

HPLC Method for Analysis mixture of Xanthinesand Uric Acid BIST B+ by SIELC Technologies
November 16, 2022
HPLC Method for Analysis mixture of Xanthines and Uric Acid BIST B+ by SIELC Technologies.
Separation type: Hydrophilic interaction liquid chromatography (HILIC)
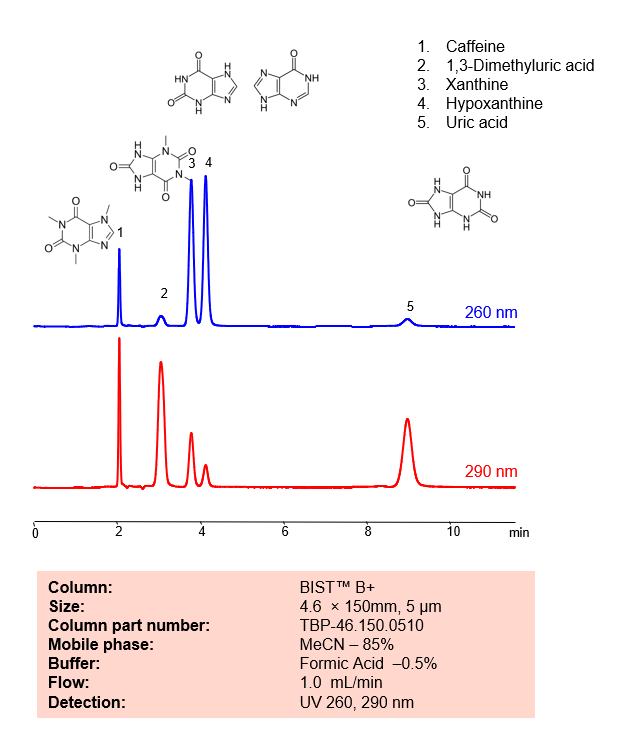
Xanthines and uric acid are related compounds in the body and both are involved in the metabolism of purines.
Xanthines are a group of alkaloids that are widely distributed in plants, and also occur in the tissues and fluids of animals. They are known to stimulate the central nervous system and cardiac muscle, and also have diuretic effects. Some commonly known xanthines include caffeine (found in coffee, tea, and chocolate), theobromine (found in cocoa and chocolate), and theophylline (used as a drug in the treatment of respiratory diseases like asthma).
In the body, xanthines are intermediates in the degradation of adenosine monophosphate to uric acid. This metabolic pathway starts with adenosine monophosphate (AMP), which is deaminated to form inosine monophosphate (IMP). IMP is then converted into a xanthine known as hypoxanthine. Hypoxanthine is then oxidized to xanthine, and finally, xanthine is further oxidized to uric acid. Both of the oxidation steps are catalyzed by the enzyme xanthine oxidase.
Uric acid and Xanthines can be retained, analyzed, and separated using an isocratic analytical method on a BIST B+ column. The simple mobile phase for this method comprises water, acetonitrile (MeCN), and formic acid as an ionic modifier. The analytical method can be monitored with UV detection at 260 nm, an Evaporative Light Scattering Detector (ELSD), or any other evaporative detection method such as Charged Aerosol Detection (CAD) or Electrospray Ionization Mass Spectrometry (ESI-MS)
Condition
| Column | BIST B+, 4.6 x 150 mm, 10 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 85% |
| Buffer | FA – 0.5% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260, 290 nm |
| Peak Retention Time | 2.01, 3.02, 4.2, 9.09 min |
Description
| Class of Compounds | Acid, Xanthines |
| Analyzing Compounds | Uric Acid, Caffeine, 1,3-Dimethyluric acid, Xanthine, Hypoxanthine |
Application Column
BIST B+
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 10 µm
Pore Size: 100 A
Column options: dual ended
Caffeine
Hypoxanthine
Uric acid
Xanthine

HPLC Determination of Caffeine, Theophylline and Theobromine in Tea on Primesep SB Column
December 17, 2020
| Column | Primesep SB, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | EtOH – 10% |
| Buffer | H3PO4 – 0.3% |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Xanthine, Hydrophobic, Ionizable |
| Analyzing Compounds | Caffeine, Theophylline, Theobromine |
Application Column
Primesep SB
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsTheobromine
Theophylline

HPLC Analysis of Caffeine in Drinks with Environmentally Friendly Mobile Phase on Primesep SB Column
December 14, 2020
| Column | Primesep SB, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | EtOH – 10% |
| Buffer | H3PO4 – 0.3% |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Xanthine, Hydrophobic, Ionizable |
| Analyzing Compounds | Caffeine |
Application Column
Primesep SB
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options
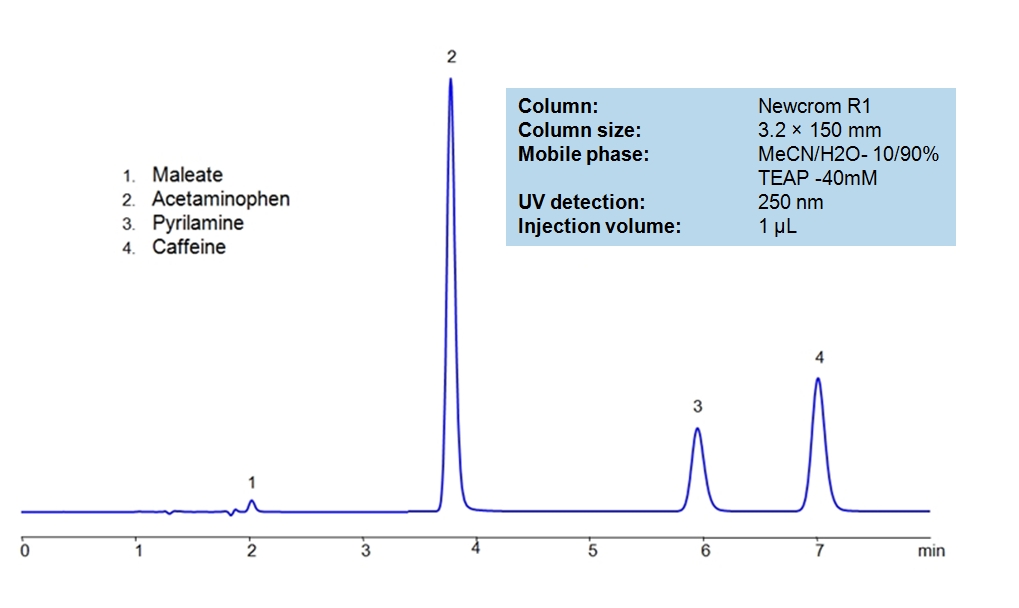
HPLC Separation of Acetaminophen, Caffeine and Pyrilamine maleate
March 2, 2018
Acetaminophen is a p-aminophenol derivative with analgesic and antipyretic activities. It has weak anti-inflammatory properties, may cause liver, blood cell, and kidney damage. It is a nonprescription medication for mild-to-moderate pain and fever. Caffeine is a Central Nervous System (CNS) stimulant. It is an unregulated and legal drug in most parts of the world. It can be found in the seeds and leaves in a number of plants native in Africa, East Asia and South America. Pyrilamine maleate is a histamine H1 antagonist with hypnotic properties. It has multiple uses such as an anesthetic and is used against allergies. Newcrom R1, a column that takes advantage of the newest technologies, does not contain embedded acidic nor basic ionizable groups and can retain Acetaminophen, Caffeine and Pyrilamine maleate. The method is UV compatible and can be used as a general approach for analyzing similar compounds.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsCaffeine
Maleate
Pyrilamine
Pyrilamine maleate

HPLC Separation of Caffeine, Benzoic acid and Acetaminophen
August 6, 2015
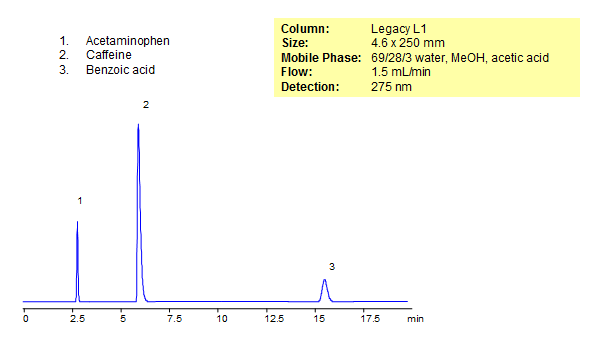
| Column | Legacy L1, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeOH – 28% |
| Buffer | Acetic Acid – 3% |
| Flow Rate | 1.5 ml/min |
| Detection | UV, 275 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Hydrophobic, Ionizable |
| Analyzing Compounds | Caffeine, Benzoic acid, Acetaminophen |
&
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select optionsBenzoic Acid
Caffeine

USP Methods for the Analysis of an Analgesic Mixture Using the Legacy L1 Column
June 21, 2012
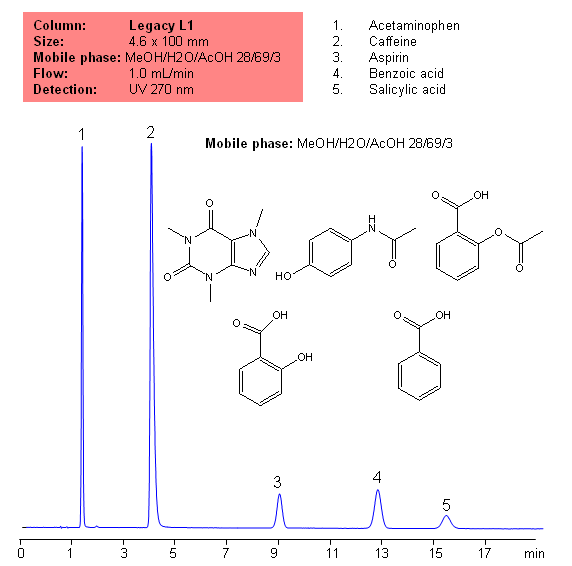
Application Notes: Acetametaphin, aspirin, and caffeine tablets contain not less than 90 percent and not more than 110 percent of the labeled amounts if acetametaphin, asprin, and caffeine according the USP methods. USP HPLC method for separation of acetaminophen, aspirin and caffeine was developed on Legacy L1 column according to US Pharmacopeia methodology. L1 classification is assigned to reversed-phase HPLC column contains C18 ligands. Support for the material is a spherical silica gel with particles size 3-10 um and pore size of 100-120A. Resolution between critical pairs corresponds to rules and specifications of USP.
Application Columns: Legacy L1 C18 HPLC column
Application compounds: Acetaminophen, Aspirin, Caffeine, benzoic acid, and salicylic acid
Mobile phase: MeOH/H2O/AcOH 28/69/3
Detection technique: UV
Reference: USP30: NF35
| Column | Legacy L1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeOH/H2O/AcOH 28/69/3 |
| Buffer | AcOH |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophobic, Ionizable |
| Analyzing Compounds | Acetaminophen, Caffeine, Aspirin, Benzoic acid, Salicylic acid |
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select optionsAspirin
Benzoic Acid
Caffeine

USP Methods for the Analysis of Caffeine using the Legacy L1 Column
June 21, 2012
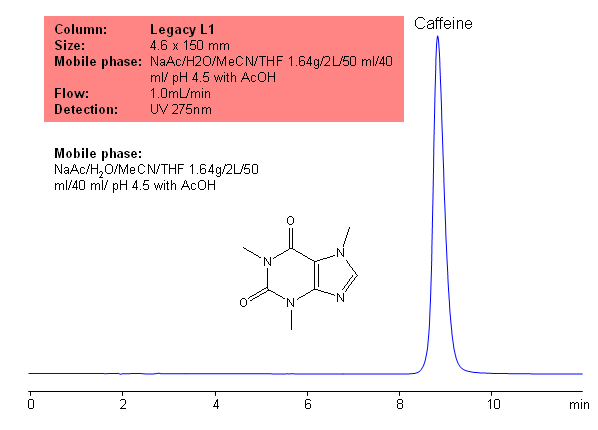
Application Notes: Caffeine is the most common stimulant used. According to USP methods, caffeine should be anhydrous or contain no more than one molecule of water of hydration. Additionally, caffeine should not contain more than 101% and no less 98.5% caffeine calculate on a anhydrous basis. The USP HPLC method for the separation of caffeine was developed on Legacy L1 column according to the US Pharmacopeia methodology. L1 classification is assigned to reversed-phase HPLC column containing C18 ligand. Support for the material is spherical silica gel with particles size 3-10 um and pore size of 100-120A. Resolution between critical pairs corresponds to rules and specifications of UPS.
Application Columns: Legacy L1 C18 HPLC column
Application compounds: Caffeine
Mobile phase: NaAc/H2O/MeCN/THF 1.64g/2L/50 ml/40 ml/ pH 4.5 with AcOH
Detection technique: UV
Reference: USP35: NF30
Application Columns: Legacy L1 C18 HPLC column
Application compounds: Hydrocortisone
Mobile phase: MeCN/H2O 25:75
Detection technique: UV
Reference: USP30: NF35
| Column | Legacy L1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | NaAc/H2O/MeCN/THF 1.64g/2L/50 ml/40 ml/ pH 4.5 with AcOH |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 275 nm |
| Class of Compounds |
Xanthine, Hydrophobic, Ionizable |
| Analyzing Compounds | Caffeine |
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select options
HPLC Separation of Caffeine, 3- Methylxanthine, 1- Methylxanthine, Xanthine
June 15, 2012
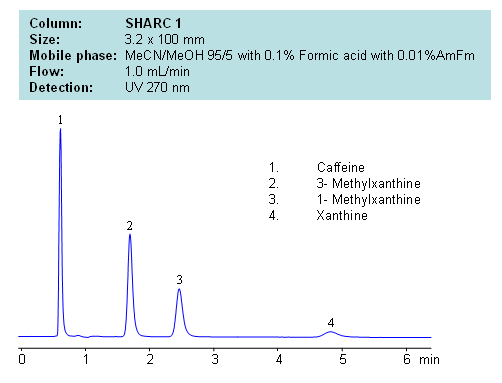
Application Notes: Xanthines are polar neutral compounds which are hard to retain and separate by traditional reversed-phase chromatography. However a hydrogen bonding method makes separation possible due to an observable correlation between the number of hydrogens available for interaction and retention time. Molecules with no hydrogens available for interactions retain less, and compound with multiple hydrogen donors retain the most. Retention time can be controlled by changing ratio of ACN:MeOH. Other protic and aprotic solvents can be used to control retention time and selectivity of separation.
Application Columns: SHARC 1, 3.2×100 mm, 5 um, 100A, To learn more about SHARC 1 columns click here. To order this column click here. To see more chromatographic separations check our web site.
Application Compounds: Caffeine, 3-methylxanthine, 1-methylxanthine, and xanthine
| Column | Sharc 1, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH |
| Buffer | AmFm, Formic acid |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Caffeine, 3- Methylxanthine, 1- Methylxanthine, Xanthine |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select options3-Methylxanthine
Caffeine
Xanthine

HPLC Separation of Caffeine and Phenylephrine on Primesep 200
October 14, 2010
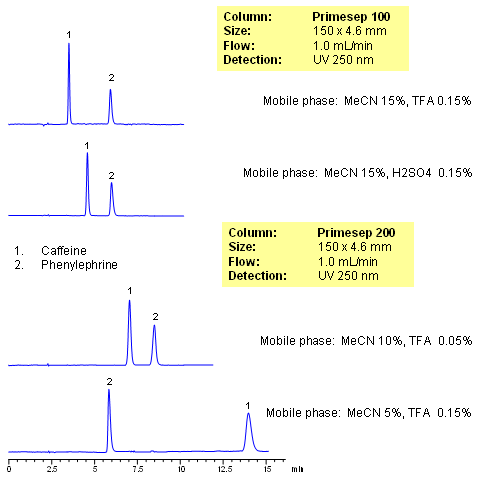
Caffeine and phenylephrine are some of the components of various pain killer/fever reducers/cough compositions. In this application, both of these compounds are separated on Primesep 100 and Primesep 200 columns. Caffeine is retained by reversed-phase mechanisms and phenylephrine is retained by combination of reversed-phase and cation-exchange mechanisms. Retention time for caffeine is controlled by the amount of acetonitrile, while retention time of phenylephrine is controlled by amount of the acetonitrile, buffer concentration and buffer pH. The method can be used for analysis of components of pain, cough and cold medication in pharmaceutical production. Method is robust and reproducible and provides good retention and peak shape. Compounds are monitored by UV, ELSD, CAD or LC/MS. Analysis of active components on biofluids (urine, plasma, blood, etc) is possible with additional sample preparation (protein precipitation, SPE, etc.)
Application Column
Primesep 200
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Phenylephrine

HPLC Separation of Caffeine and Phenylephrine on Primesep 100
October 14, 2010

Caffeine and phenylephrine are some of the components of various pain killer/fever reducers/cough compositions. In this application, both of these compounds are separated on Primesep 100 and Primesep 200 columns. Caffeine is retained by reversed-phase mechanisms and phenylephrine is retained by combination of reversed-phase and cation-exchange mechanisms. Retention time for caffeine is controlled by the amount of acetonitrile, while retention time of phenylephrine is controlled by amount of the acetonitrile, buffer concentration and buffer pH. The method can be used for analysis of components of pain, cough and cold medication in pharmaceutical production. Method is robust and reproducible and provides good retention and peak shape. Compounds are monitored by UV, ELSD, CAD or LC/MS. Analysis of active components on biofluids (urine, plasma, blood, etc) is possible with additional sample preparation (protein precipitation, SPE, etc.)
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Phenylephrine

HPLC Analysis of Active Drug in a Formulation
October 4, 2010
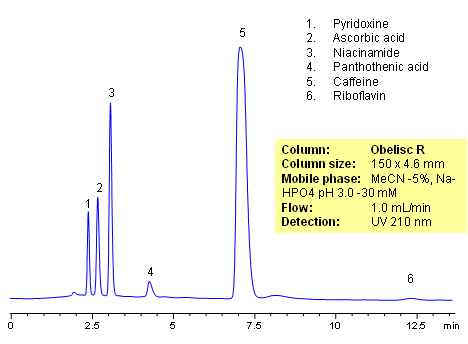
HPLC method for separation of active ingredients of drug/supplemental composition was developed on an Obelisc R trimodal HPLC column. Compounds are retained by combination of reversed-phase, cation-exchange and anion-exchange mechanisms. Compounds are well separated, and method can be used for quantitation of pyridoxine, ascorbic acid, niacinamide, pantothenic acid, caffeine and riboflavin in a mixture or as separate compounds in various complex mixtures. Various detection techniques can be applied for quantitation (ELSD, UV, LC/MS, Corona). This HPLC method can be adopted as general approach for analysis of active drug components in various formulations.
| Column | Obelisc R , 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O -5/95% |
| Buffer | NaHPO4 pH 3.0 – 30 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Vitamin B₆, Hydrophobic, Ionizable |
| Analyzing Compounds | Pyridoxine, Ascorbic acid, Niacinamide, Pantothenic acid, Caffeine, Riboflavin |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsAscorbic Acid
Caffeine
Niacinamide
Pantothenic Acid
Vitamin B6 (Pyridoxine)

HPLC Separation of Components of Excedrin (Benzoic acid, Acetaminophen, Caffeine, Aspirin)
July 16, 2009
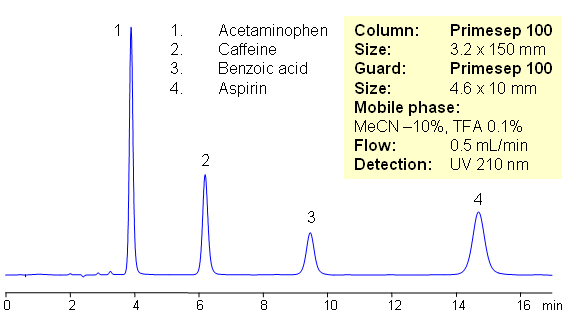
Excedrin is over-the-counter pain reliever containing acetaminophen, caffeine and aspirin as active ingredients of this drug composition. Acetaminophen (paracetamol) is used as analgesic and pain reliever. It is a neutral compound with low hydrophobicity. Aspirin or acetylsalicylic acid is used as analgesic and anti-inflammatory component of many OTC compositions. It is weakly acidic and slightly hydrophobic compound. Caffeine is xanthine alkaloid which is psychoactive stimulant drug. All four compounds are separated on mixed-mode Primesep 100 HPLC column with acetonitrile/water/TFA mobile phase. In this HPLC application compounds are retained by reversed phase mechanism. This HPLC method is short and robust.
| Column | Primesep 100, 3.2×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | TFA |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Benzoic acid, Acetaminophen, Caffeine, Aspirin |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAcetylsalicylic Acid
Aspirin
Benzoic Acid
Caffeine
UV Detection

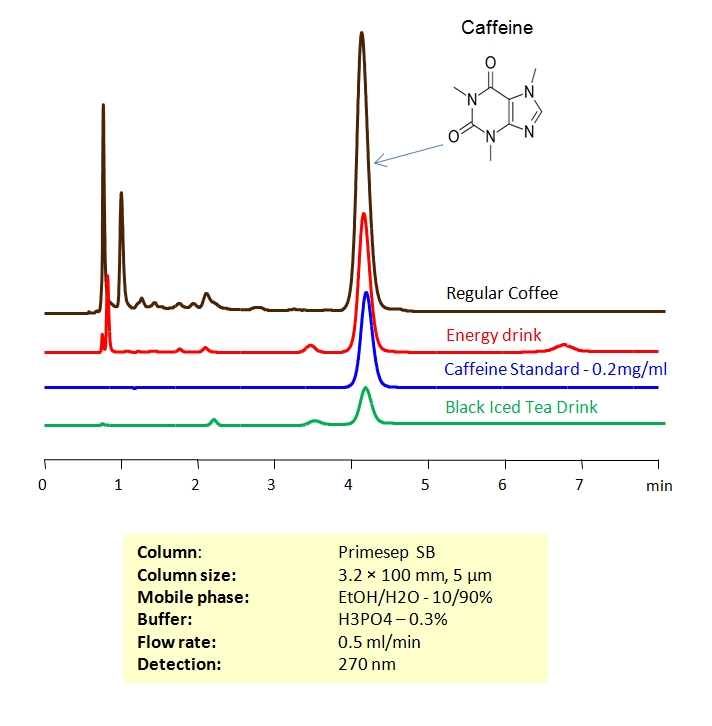
HPLC Separation of Coffee
November 6, 2006
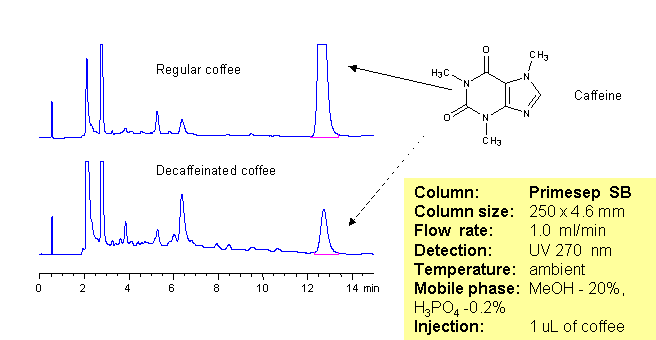
The amount of caffeine in regular and decaffeinated coffee can be determined using a Primesep SB column. The HPLC method combines reversed-phase and polar interactions to elute caffeine without interference from the coffee complex mixture. This method can be made even more robust by incorporating a diverter valve and a guard column to prevent late eluting components from sticking to the Primesep SB column. The sample is injected onto the guard column and after a defined time point, the eluent flow is reversed to elute the caffeine peak onto the analytical column without the late eluters that can shorten column life. The HPLC separation uses a mobile phase of water, methanol (MeOH) and phosphoric acid (H3PO4) and UV detection at 270 nm.
Application Column
Primesep SB
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDecaffeinated Coffee
Regular Coffee

Control of Cation Exchange Properties by pH of Primesep C Column
October 12, 2005
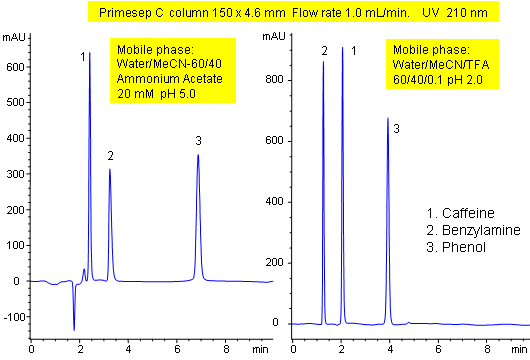
Primesep C demonstrates the control of ion-exchange properties of Primesep columns. The peak order of caffeine and benzylamine in a mixture of caffeine, benzylamine, and phenol reverses when the mobile phase modifier is changed from ammonium acetate to trifluoroacetic acid. Control of the separation is possible even with mass spec (LC/MS) compatible mobile phases of water, acetonitrile (MeCN, ACN) and ammonium acetate or trifluoroacetic acid (TFA).
Application Column
Primesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsCaffeine
Phenol