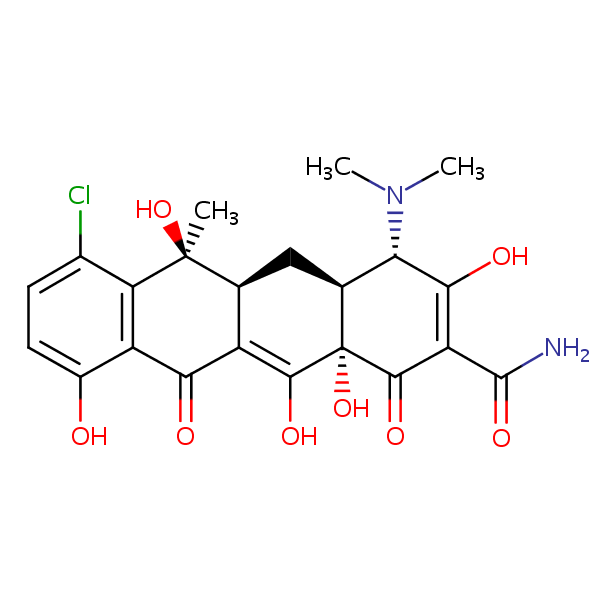
| CAS Number | 57-62-5 |
|---|---|
| Molecular Formula | C22H23ClN2O8 |
| Molecular Weight | 478.881 |
| InChI Key | CYDMQBQPVICBEU-XRNKAMNCSA-N |
| LogP | -0.620 |
| Synonyms |
|
Applications:
Separation of Antibiotics in Mixed-mode chromatography
May 11, 2015
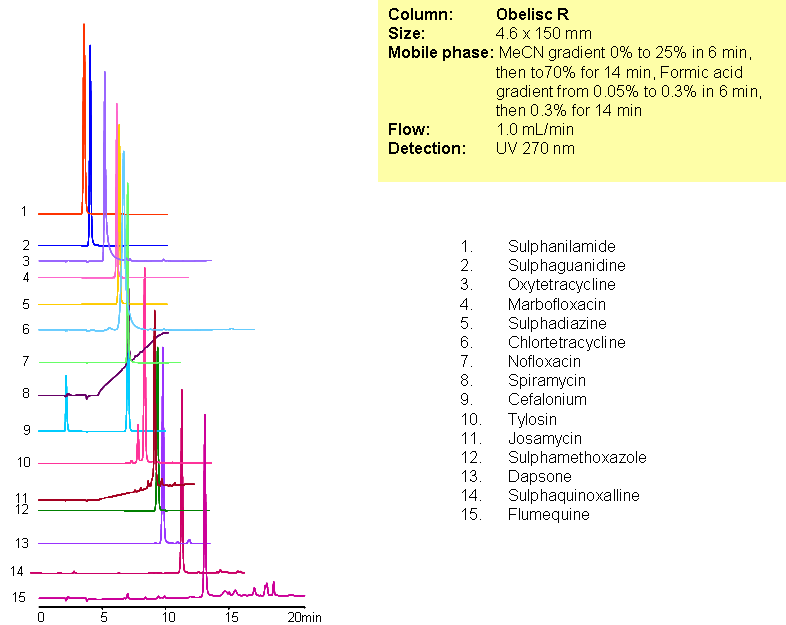
A complex mixture of sulphonamide, macrolide, tetracycline and fluoroquinolone antibiotics were separated in one run using mixed-mode chromatography with LC/MS -compatible conditions. All compounds are separated based on reversed-phase and/or ion-exchange mechanism. Method can be used for analysis of various classes of antibiotics and related impurities in different sample matrices (blood, urine, soil, waste water).
| Column | Obelisc R, 2.1×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 0-25%, 6 min, 25-70% 14 min |
| Buffer | Gradient Formic Acid – 0.05%-0.3%, 10 min, 14 min hold |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Antibiotic, Drug, Hydrophobic, Ionizable |
| Analyzing Compounds | Sulphanilamide, Sulphaguanidine, Oxytetracycline, Marbofloxacin, Sulphadiazine, Chlortetracycline, Nofloxacin, Spiramycin, Cefalonium, Tylosin, Josamycin, Sulphamethoxazole, Dapsone, Sulphaquinoxalline, Flumequine |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsChlortetracycline
Dapsone
Flumequine
Josamycin
Marbofloxacin
Norfloxacin
Oxytetracycline
Spiramycin
Sulfamethoxazole
Sulfonamides
Sulphadiazine
Sulphaguanidine
Sulphanilamide
Sulphaquinoxaline
Tetracycline
Tylosin
UV Detection

Separation of Oxytetracycline and Chlortetracycline in Mixed-Mode
May 11, 2015
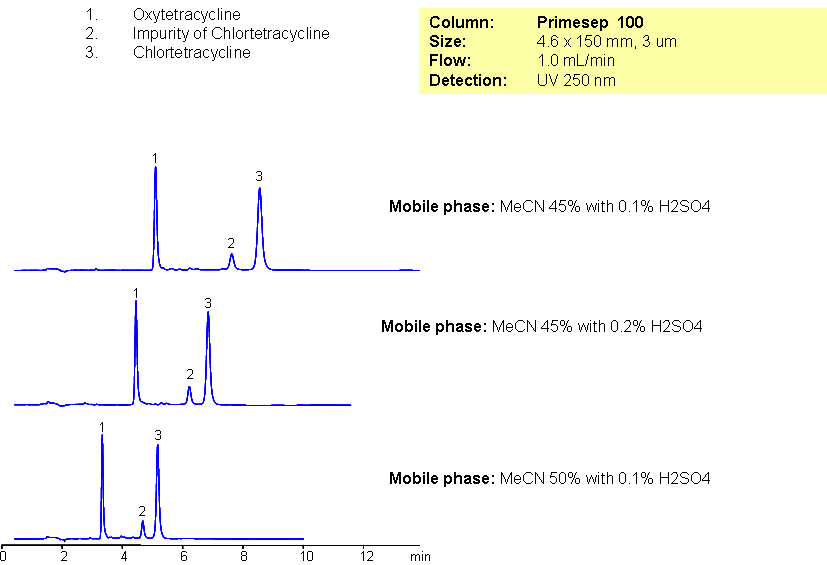
Oxytetracycline and chlortetracycline are polar compounds related to broad-spectrum of tetracycline group of antibiotics. Slight hydrophobicity and presence of the basic group in both compounds allow to employ mixed-mode reversed-phase cation-exchange mechanism for separation of these compounds. Method can be used for analysis of antibiotics and related impurities with LC/MS compatible conditions.
| Column | Primesep 100, 4.6×150 mm, 3 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Oxytetracycline, Chlortetracycline |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsOxytetracycline
UV Detection