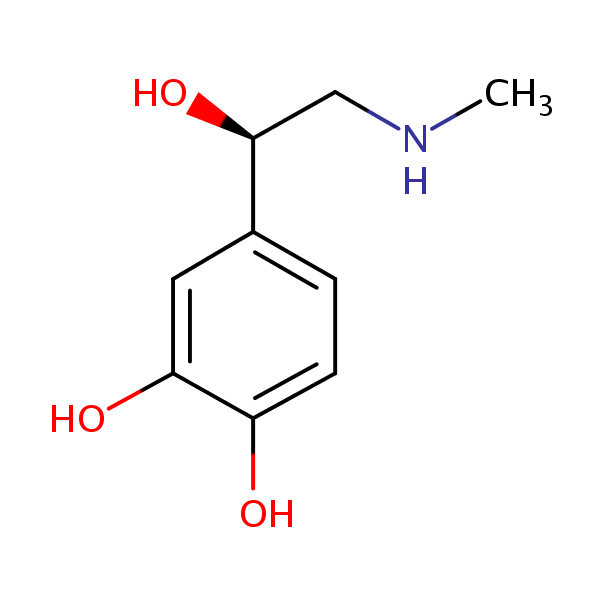
| CAS Number | 51-43-4 |
|---|---|
| Molecular Formula | C9H13NO3 |
| Molecular Weight | 183.208 |
| InChI Key | UCTWMZQNUQWSLP-VIFPVBQESA-N |
| LogP | -1.20 |
| Synonyms |
|
Applications:
HPLC Method for Analysis of Neurotransmitters Phenylephrine, Epinephrine and Norepinephrine on BIST™B+ Column
July 14, 2022
| Separation type: Bridge Ion Separation Technology, or BIST™ | ||||||||||||||
| BIST™ Ionic Modifier Preparation | ||||||||||||||
| High Performance Liquid Chromatography (HPLC) Method for Analysis of Phenylephrine, Epinephrine, Norepinephrine
Epinephrine and norepinephrine are key neurotransmitters and adrenal hormones naturally produced by the body to generate the fight-or-flight response, and synthesized by pharmaceutical companies to treat a host of issues, such as allergic reactions and low blood pressure. Phenylephrine is a related, synthetic compound made to treat low blood pressure, congestion, and hemorrhoids. Using SIELC’s newly introduced BIST™ method, these three similar neurotransmitters, which protonate in water, can be retained on a positively-charged anion-exchange BIST™ B+ column. There are two keys to this retention method: 1) a multi-charged, negative buffer, such as Sulfuric acid (H2SO4), which acts as a bridge, linking the positively-charged amine analytes to the positively-charged column surface and 2) a mobile phase consisting mostly of organic solvent to minimize the formation of a solvation layer around the charged analytes. Using this new and unique analysis method, Epinephrine, norepinephrine, and phenylephrine can be retained and UV detected at 200 nm.
|
||||||||||||||
|
Application Column
BIST B+
BIST™ columns offer a unique and effective way to achieve separations that were traditionally challenging or even impossible with other HPLC columns. With the use of a special mobile phase, these ion exchange columns provide very strong retention for analytes with the same charge polarity as the stationary phase, unlocking new chromatography applications. What makes BIST™ columns stand out is their proprietary surface chemistry, which results in superior selectivity, resolution, and sensitivity. These columns offer a simple, efficient solution for a variety of analytical challenges, making them an excellent choice for researchers and analysts across many different fields. To learn more about the technology that powers BIST™ columns and to explore related applications, check out https://BIST.LC.
Select optionsNorphenylephrine
Phenylephrine
Phenylephrine hydrochloride

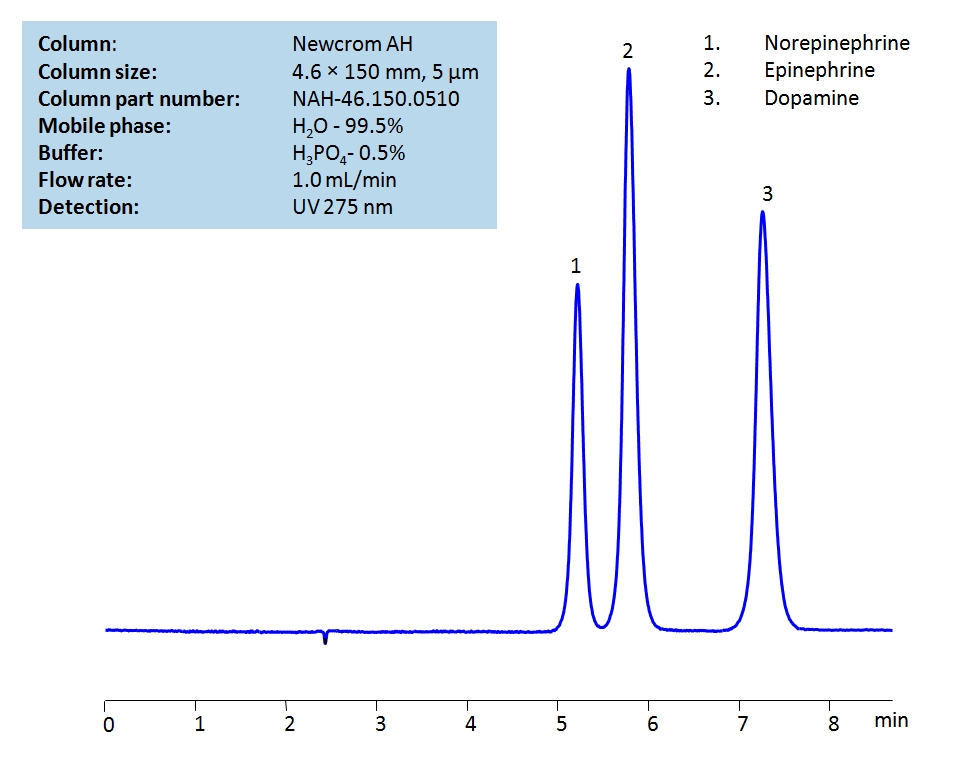
HPLC Separation of Catecholamines on Newcrom AH Column
May 20, 2021
Separation type: Liquid Chromatography Mixed-mode
Catecholamines are neurotransmitters that generate the ‘fight-or-flight’ responsein the body. The 3 main catecholamines are epinephrine (also known as adrenaline), norepinephrine, and dopamine, and they are produced by the adrenal glands.
These 3 catecholamines can be detected in the low UV regime. Using a Newcrom AH mixed-mode column and a mobile phase consisting of almost entirely water with either a phosphoric (H3PO4) acid or ammonium formate (AmFm) buffer, the catecholamines can be retained, separated, and measured. This analysis method can be UV detected at 275 nm with high resolution. The latter mobile phase (utilizing AmFm) is compatible with Mass Spectrometry.
| Column | Newcrom AH, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | H2O |
| Buffer | H3PO4 or AmFm pH 3.0 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 275 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Norepinephrine, Epinephrine, Dopamine |
Application Column
Newcrom AH
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsEpinephrine
Norepinephrine

Generic Screening Method for Complex Mixtures
October 15, 2015
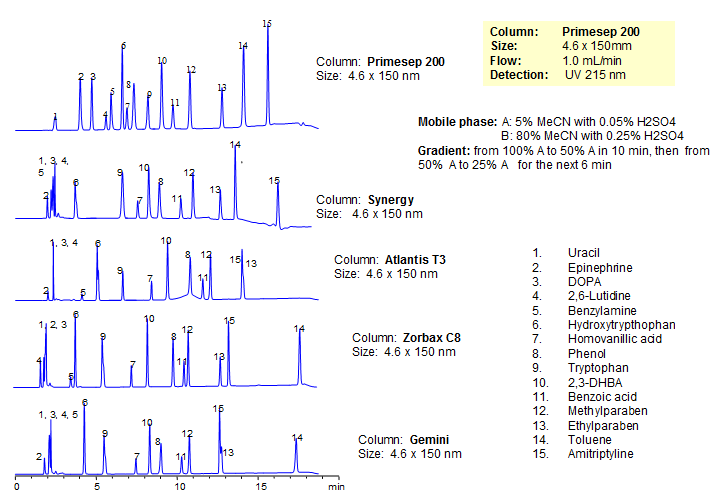
| Column | Primesep 200, 4.6*150 mm 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 215 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Uracil, Epinephrine, DOPA, 2,6-Lutidine, Benzylamine, Hydroxytrypthophan, Homovanillic acid, Phenol, Tryptophan , 2,3-DHBA, Benzoic acid, Methylparaben, Ethylparaben, Toluene, Amitriptyline |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options2,6-Lutidine
Amitriptyline
Benzoic Acid
Benzylamine
DOPA (3,4-dihydroxy-L-phenylalanine)
Epinephrine
Ethylparaben
Homovanillic Acid
Hydroxytryptophan
Methylparaben
Phenol
Toluene
Tryptophan
Uracil

HPLC Analysis of Epinephrine and Epinephrine Sulfonate on Primesep AB Column
May 12, 2015
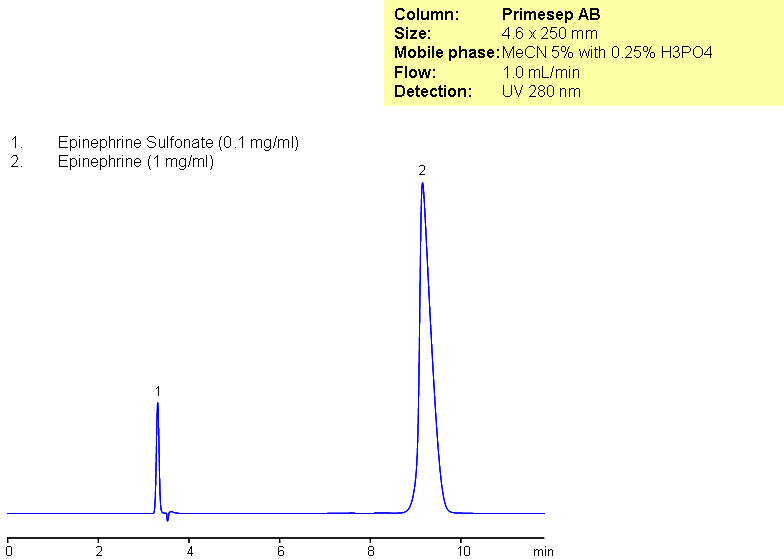
Epinephrine and epinephrine sulfonate were analyzed using Primesep AB, a reverse-phase column with both acidic and basic embedded ion-pairing groups. This method is LC/MS compatible.
| Column | Primesep AB, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 5/95% |
| Buffer | H3PO4 – 0.25% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 280 nm |
| Class of Compounds |
Drug, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Epinephrine, Epinephrine Sulfonate |
Application Column
Primesep AB
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep A
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsEpinephrine Sulfonate
UV Detection

HPLC Separation of Epinephrine on Legacy L1 Column
July 2, 2013
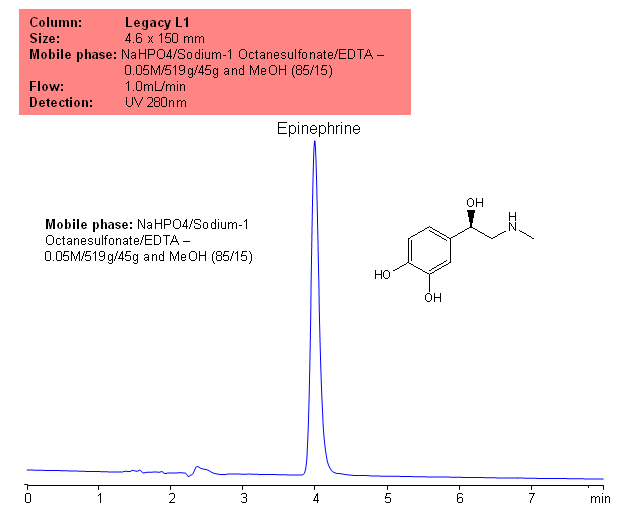
Epinephrine was analyzed according to the US Pharmacopeia method on traditional reversed-phase HPLC columns, classified as a L1 type of column according to USP classification. Legacy L1 column can be used for the analysis of drug substances in various formulations. Legacy L1 can be used in new HPLC methods as well as in validated methods which require experiments based on USP manuscript. The mobile phase can be modified to accommodate other detection techniques, like LC/MS.
| Column | Legacy L1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeOH – 15% |
| Buffer | Sodium 1 -Octane sulfonate/EDTA |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 280 nm |
| Class of Compounds |
Drug, Neurotransmitter, Hydrophobic, Ionizable |
| Analyzing Compounds | Epinephrine |
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select options
USP Methods for the Analysis of Epinephrine Using the Legacy L1 Column
June 21, 2012
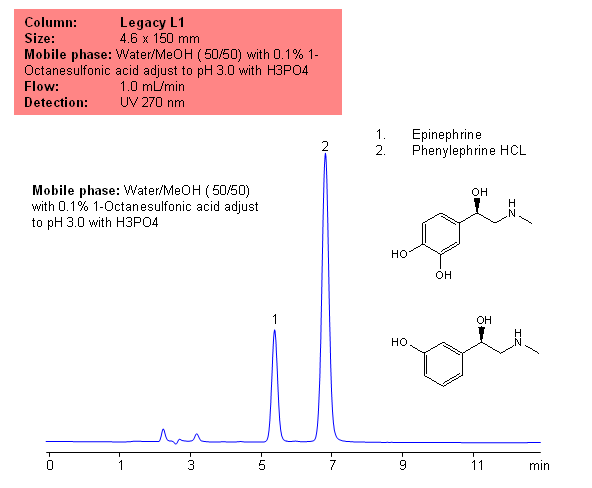
Application Notes: Epinephrine is a synthetic adrenaline used to treat cardiac arrest and anaphylaxis. Phenylephrine is a decongestant and is often used instead of pseudoephedrine. According to the USP methods epinephrine contains no less than 97% and no more than 100.5 percent of epinephrine calculated on a dried basis. The USP HPLC method for the separation of phenylephrine and epinephrine was developed on Legacy L1 column according to the US Pharmacopeia methodology. L1 classification is assigned to reversed-phase HPLC column containing C18 ligand. Support for the material is spherical silica gel with particles size 3-10 um and pore size of 100-120A. Resolution between critical pairs corresponds to rules and specifications of USP.
Application Columns: Legacy L1 C18 HPLC column
Application compounds: Epinephrine and Phenylphrine
Mobile phase: Water/MeOH (50/50) with 1% 1-Octanesulfonic acid adjust to pH 3.0 with H3PO4
Detection technique: UV
Reference: USP35: NF30
| Column | Legacy L1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeOH/MeOH – 50/50% |
| Buffer | 1-Octanesulfonic acid adjust to pH 3.0 with H3PO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Antiarrhythmic, Hydrophobic, Ionizable |
| Analyzing Compounds | Epinephrine |
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select options
HPLC Separation of Dopamine and Epinephrine in HILIC and Cation-Exchange Modes
July 14, 2011
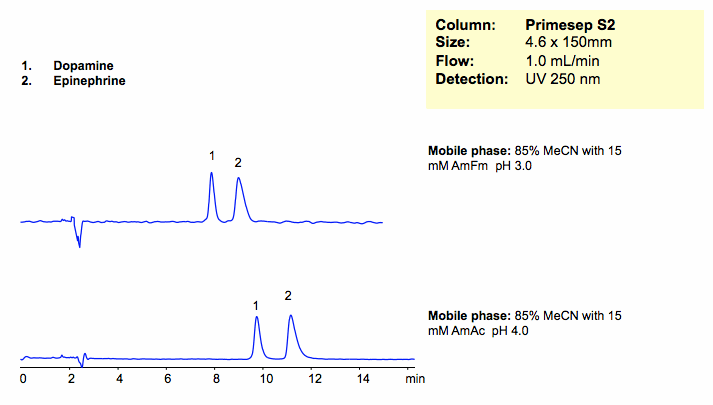
Dopamine and epinephrine were separated on a Primesep S2 HILIC mixed-mode column. Dopamine and epinephrine are retained by HILIC and cation-exchange mechanisms. Retention time is controlled by the amount of ACN, buffer and buffer pH. Method is compatible with LC/MS and can be used for analysis of polar metabolites in biofluids.
| Column | Primesep S2, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Monocarboxylic acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Dopamine, Epinephrine |
Application Column
Primesep S2
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsEpinephrine

HPLC Separation of Catecholamines on Primesep 100 Column with Phosphate Buffers
July 8, 2011
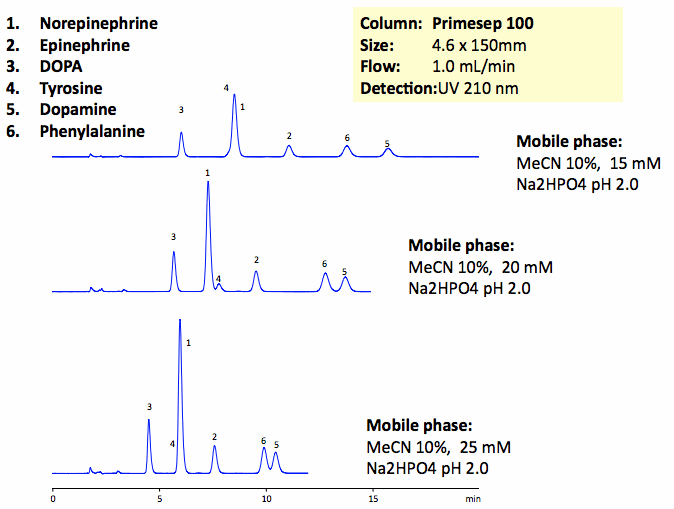
The catecholamine neurotransmitters are amino-acid derivatives of tyrosine. DOPA, tyrosine, phenylalanine, norepinephrine, epinephrine, and dopamine and baseline are resolved on a Primesep 100 column with UV-transparent phosphate buffer. This method can be used for analysis of catecholamines and related impurities in various matrices. Peak order and retention time can be changed by changing the amount of ACN, buffer concentration and buffer pH. Various buffers can be used to accommodate desired detection technique. Primesep 100 is a reversed-phase cation-exchange mixed-mode column that can be used for analysis of polar neutral, polar ionizable, polar zwitter-ionic, hydrophobic neutral, and hydrophobic ionic compounds in the same run. Column can be operated in reverse-phase, cation-exchange, anion-exclusion, HILIC and mixed-modes depending on the mobile phase selection and nature of analytes. Column is compatible with LC/MS and does not require use of ion-pairing reagents.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | Na2HPO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Tyrosine, DOPA, Phenylalanine, Norepinephrine, Epinephrine, Dopamine |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDopamine
Epinephrine
Norepinephrine
Phenylalanine
Tyrosine

HPLC Separation of Epinephrine and Epinephrine Sulfonate
June 5, 2011
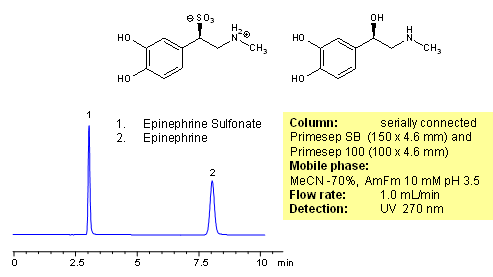
Epinephrine and epinephrine sulfonate are separated on combination of Primesep 100 and Primesep SB HPLC columns. Primesep 100 column retains epinephrine by combination of reversed-phase and cation-exchange mechanisms. Epinephrine sulfonate is retained by weak reversed-phase mechanism. Due to strong zwitter-ionic nature of epinephrine sulfonate, ionic interaction is not available for this molecule. Compounds can be monitored by UV, ELSD, CAD or LC/MS.
| Column | Primesep SB, Primesep 100 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 70/30% |
| Buffer | AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Epinephrine, Epinephrine Sulfonate |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep SB
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsEpinephrine Sulfonate
UV Detection

HPLC Separation of Epinephrine and Related Impurities
July 16, 2009
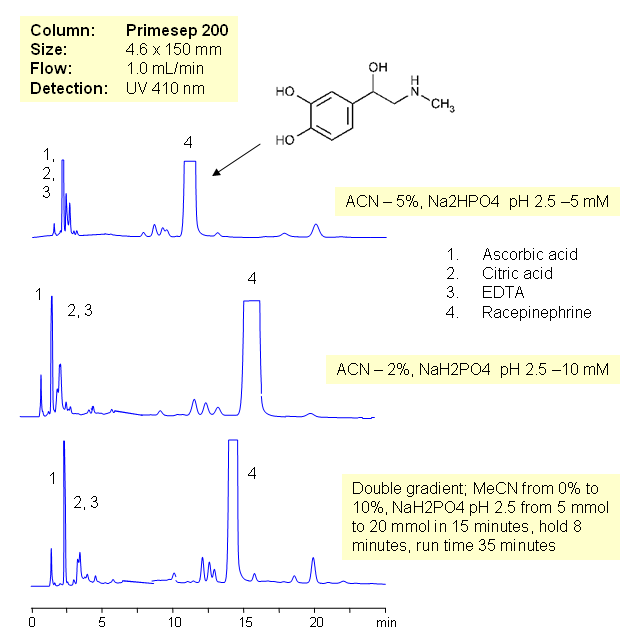
Epinephrine (also referred to as adrenaline) is a hormone and neurotransmitter. It is a catecholamine, a sympathomimetic monoamine derived from the amino acids phenylalanine and tyrosine. Epinephrine is polar basic compounds and it is retained on mixed-mode cation exchange columns without ion-pairing reagent. Epinephrine is retained by weak reversed phase and strong cation-exchange mechanisms. Formulations for epinephrine might have citric and ascorbic acid and EDTA. These three compounds are not retained by cation-exchange or reversed-phase mechanisms and elute in the void. Current HPLC method can be used for quantitation of epinephrine and recepinephrine (recemate) in various compositions. Epinephrine and related impurity can be monitored by UV, ELCD/MS, ELSD or Corona CAD. Corresponding buffer is required for each detection technique.
| Column | Primesep 200, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | NaH2PO4 |
| Flow Rate | 1.0 ml/min |
| Detection | 410 |
| Class of Compounds |
Ions, Hydrophilic, Ionizable |
| Analyzing Compounds | Ascorbic acid, Citric acid, EDTA, Racepinephrine |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsRacepinephrine

HILIC Separation of Epinephrine and Epinephrine Sulfonate
August 22, 2008
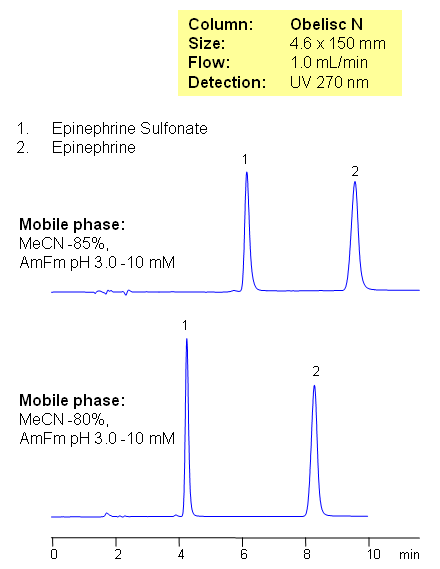
Epinephrine (also referred to as adrenaline) is a hormone and neurotransmitter. It is a catecholamine, a sympathomimetic monoamine derived from the amino acids phenylalanine and tyrosine. This method can be used to determine and quantitate epinephrine and epinephrine sulfonate in biological fluids (urine, blood, serum) and drug formulations. Obelisc N columns are used to retain and separate epinephrine and epinephrine sulfonate in mixed-mode hydrophilic interaction chromatography. Epinephrine is retained by the combination of cation-exchange and HILIC mechanisms. Epinephrine sulfonate is retained by HILIC mechanism. Buffer concentration and pH, as well as the amount of acetonitrile, can be used to adjust retention of both compounds. Both compounds can be detected by UV, ELSD and LC/MS. Preparative separation is possible with volatile mobile phases (ammonium formate or ammonium acetate.
| Column | Obelisc N, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm pH 3.0 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Epinephrine, Epinephrine Sulfonate |
Application Column
Obelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsEpinephrine Sulfonate

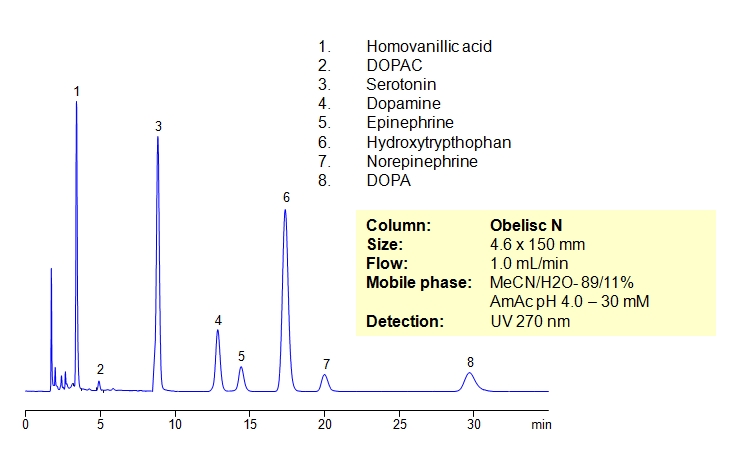
Separation of Serotonin, Dopamine, and Related Compounds
August 22, 2008
Catecholamines are chemical compounds derived from the amino acid tyrosine containing catechol and amine groups. Some of them are biogenic amines. Retention of compounds of the catecholamine pathway is achieved on Obelisc N column. All polar compounds are well retained by combination of HILIC and ion-exchange mechanisms. Obelisc N columns produce very good peak shapes for all analytes. The method is very sensitive to amount of ACN, buffer and buffer pH. The retention time changes with variation of the main parameters. This method can be used for quantitation of biogenic amines and related compounds (homovanillic acid, dihydroxyphenyl acetic acid, serotonin, dopamine, epinephrine, hydroxytryptophan, epinephrine and DOPA) in urine, blood and other biological fluids. Further optimization of this HPLC method can be used during screening and validation. Amines and acids can be analyzed in the same run and retained by a combination of polar organic mode, cation-exchange and anion-exchange modes. Various buffers within specified pH can be employed (ammonium formate, ammonium acetate, sodium phosphate, etc.).
| Column | Obelisc N, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc pH 4.0- 30 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Monocarboxylic acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Homovanillic acid, Dihydroxyphenyl acetic acid, Serotonin, Dopamine, Epinephrine, Hydroxytryptophan, DOPA |
Application Column
Obelisc N
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsDOPAC (Dihydroxyphenylacetic Acid)
Dopamine
Epinephrine
Homovanillic Acid
Hydroxytryptophan
Norepinephrine
Serotonin

HPLC Separation of Neurotransmitters and Related Drugs
August 22, 2008
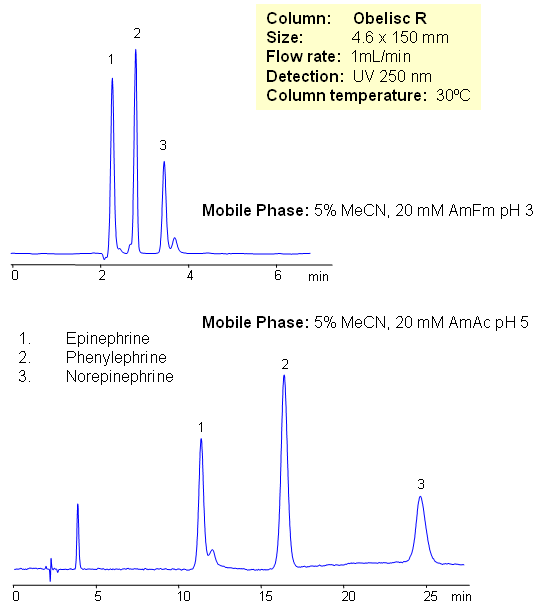
Epinephrine and norepinephrine (adrenaline and noradrenaline) are hormones and neurotransmitters. Epinephrine is synthesized from norepinephrine in a synthetic pathway shared by all catecholamines, including L-dopa, dopamine, norepinephrine, and epinephrine. Phenylephrine is used as a decongestant, available as an oral medicine or as a nasal spray. Phenylephrine is now the most common over-the-counter (OTC) decongestant. All three compounds are used in various drug compositions. Separation of epinephrine and norepinephrine is a challenging task due to polarity and close properties of two compounds. Epinephrine, norepinephrine and phenylephrine are separated in this method on Obelisc R mixed-mode HPLC columns. The method is very sensitive to variation of pH and pH adjustment can be used to achieve desired selectivity and retention time. Other catecholamines can be analyzed using this HPLC method. The method can be used as a stability indicating or a impurity profiling approach to the analysis of neurotransmitters in drug formulation, blood, serum and urine.
| Column | Obelisc R, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Phenylalanine, Norepinephrine, Epinephrine |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsNorepinephrine
Phenylephrine

HPLC Separation of Compounds of Catecholamine Pathway
March 3, 2006
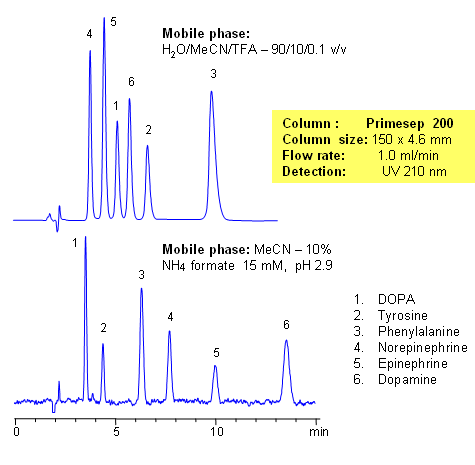
The catecholamine neurotransmitters are amino-acid derivatives of tyrosine. DOPA, tyrosine, phenylalanine, norepinephrine, epinephrine, and dopamine and baseline are resolved on a
Primesep 200 column with UV-transparent phosphate buffer. This method can be used for the analysis of catecholamines and related impurities in various matrices. The peak order and retention time can be changed by changing the amount of ACN, buffer concentration and buffer pH. Various buffers can be used to accommodate the desired detection technique. Primesep 200 is a reversed-phase cation-exchange mixed-mode column that can be used for analysis of polar neutral, polar ionizable, polar zwitterionic, hydrophobic neutral, and hydrophobic ionic compounds in the same run. Column can be operated in reverse-phase, cation-exchange, anion-exclusion, HILIC and mixed-modes depending on the mobile phase selection and the nature of the analytes. The column is compatible with LC/MS and does not require the use of ion-pairing reagents.
| Column | Primesep 200, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | TFA, AmFm |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Tyrosine, DOPA, Phenylalanine, Norepinephrine, Epinephrine, Dopamine |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDopamine
Epinephrine
Phenylalanine
Tyrosine
UV Detection

HPLC Analysis of the Catecholamine Pathway
January 17, 2004
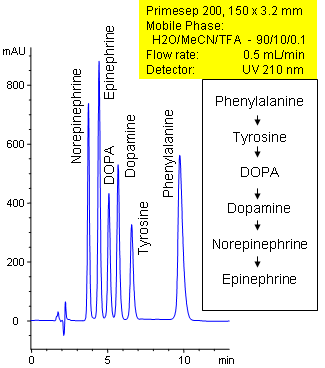
Primesep 200 separates catecholamines in the catecholamine pathway in 10 minutes. Phenylalanine, tyrosine, DOPA, dopamine, norepinephrine, and epinephrine are baseline resolved by a combination of reversed-phase, ion-exchange, and ion-exclusion mechanisms. Excellent peak shape results with a mass spec compatible mobile phase of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) with UV detection at 210 nm.
| Column | Primesep 200, 3.2×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 10/90% |
| Buffer | TFA – 0.1% |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Tyrosine, DOPA, Phenylalanine, Norepinephrine, Epinephrine, Dopamine |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDopamine
Epinephrine
Phenylalanine
Tyrosine

Separation of Epinephrine Norepinephrine
January 17, 2004
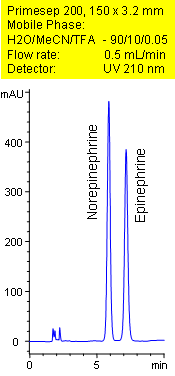
Primesep 200 separates the catecholamines, norepinephrine and epinephrine, less than 8 minutes. Norepinephrine and epinephrine are baseline resolved by a combination of reversed-phase, and ion-exchange mechanisms. Excellent peak shape results with a mass spec compatible mobile phase of water, acetonitrile (MeCN, ACN) and trifluoroacetic acid (TFA) with UV detection at 210 nm.
| Column | Primesep 200, 3.2×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 10/90% |
| Buffer | TFA – 0.05% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Norepinephrine, Epinephrine |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsNorepinephrine

HPLC Separation of Catecholamines
January 8, 2004
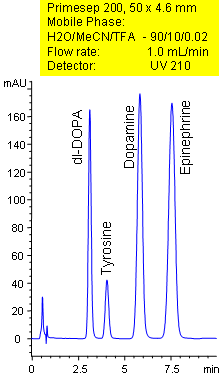
Primesep 200 separates catecholamines in less than 8 minutes. Tyrosine, dl-DOPA, dopamine, epinephrine are baseline resolved by a combination of reversed-phase, ion-exchange, and ion-exclusion mechanisms. Excellent peak shape results with a mass spec compatible mobile phase of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) with UV detection at 210 nm.
| Column | Primesep 200,4.6×50 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | TFA |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Tyrosine, DOPA, Epinephrine, Dopamine |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsEpinephrine