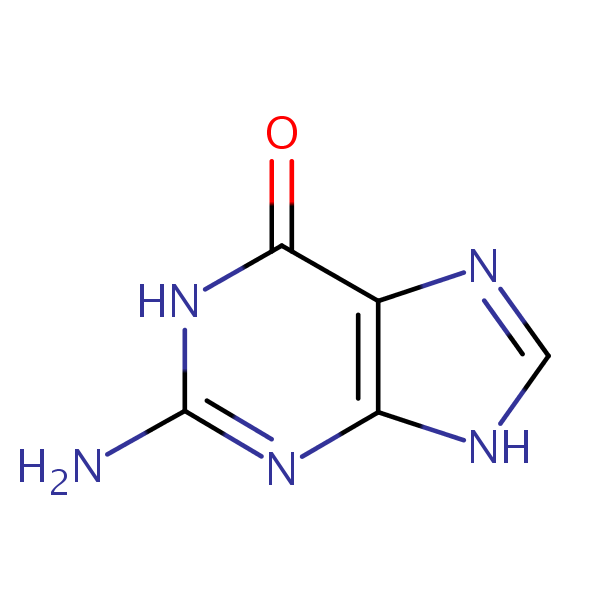
| CAS Number | 73-40-5 |
|---|---|
| Molecular Formula | C5H5N5O |
| Molecular Weight | 151.129 |
| InChI Key | UYTPUPDQBNUYGX-UHFFFAOYSA-N |
| LogP | -0.910 |
| Synonyms |
|
Applications:
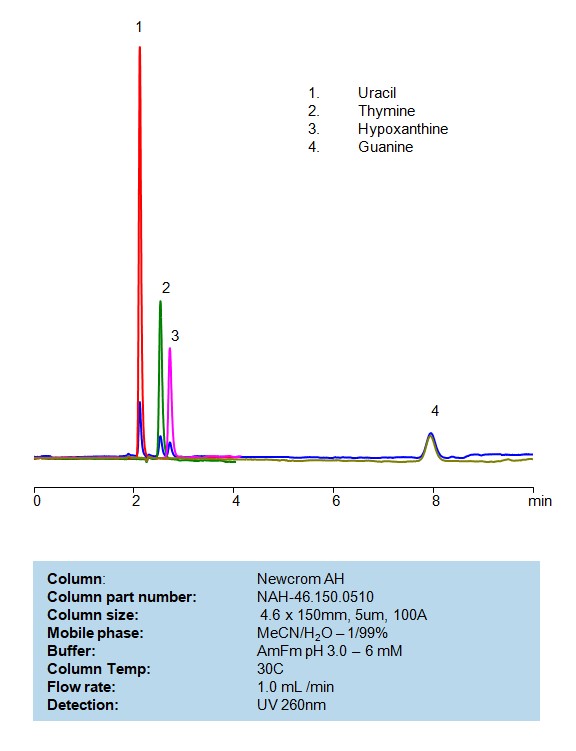
HPLC Separation of Uracil, Thymine, Hypoxanthine and Guanine on Newcrom AH
October 31, 2023
HPLC Method for Analysis of Uracil, Thymine, Hypoxanthine, Guanine on Newcrom AH Column by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
Uracil, Thymine, Hypoxanthine, and Guanine are all nitrogenous bases, each playing distinct roles in the biochemistry of nucleic acids.
Uracil (U):
- Found in: RNA
- Pairs with: Adenine (A)
- Structure: Pyrimidine
- Role: Uracil replaces thymine in RNA. In DNA, adenine pairs with thymine, but in RNA, adenine pairs with uracil.
Thymine (T):
- Found in: DNA
- Pairs with: Adenine (A)
- Structure: Pyrimidine
- Role: Thymine is specific to DNA, distinguishing it from RNA. It’s the base that pairs with adenine through two hydrogen bonds.
Hypoxanthine:
- A naturally occurring purine derivative. It’s not directly a base in standard DNA or RNA, but it plays a significant role in the metabolism of purines.
- It is the base form of the nucleoside inosine, which can be found in certain tRNAs and plays a role in wobble base pairing.
- Hypoxanthine is also an intermediate in the purine degradation pathway, leading to the production of uric acid.
Guanine (G):
- Found in: Both DNA and RNA
- Pairs with: Cytosine (C)
- Structure: Purine
- Role: Guanine is one of the four main nucleobases in the nucleic acids DNA and RNA. It forms three hydrogen bonds with cytosine, contributing to the stability of the nucleic acid structures.
These nitrogenous bases are crucial for the structure, replication, and function of nucleic acids. Understanding their properties and interactions is fundamental to molecular biology and genetics.
Nucleotides can be retained and analyzed on a mixed-mode Newcrom AH column with a mobile phase consisting of water, Acetonitrile (MeCN), and ammonium formate. This analytical method can detect compounds with high resolution and peak symmetry using UV detection at 260 nm
High Performance Liquid Chromatography (HPLC) Method for Analyses of Uracil, Thymine, Hypoxanthine and Guanine
Condition
| Column | Newcrom AH, 4.6 x 150 mm, 5 µm, 100 A |
| Mobile Phase | MeCN – 1% |
| Buffer | Ammonium formate pH 3.0- 6 mM |
| Flow Rate | 1.0 ml/min |
| Peak Retention Time | 2.21, 2.38, 2.81, 7.92 min |
| Detection | 260 nm |
Description
| Class of Compounds | Nucleotides |
| Analyzing Compounds | Uracil, Thymine, Hypoxanthine, Guanine |
Application Column
Newcrom AH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Hypoxanthine
Thymine
Uracil

Ionic Modifier Effect on Selectivity of Separation of Deoxyguanosine, Guanine and Guanosine on BIST B+
December 5, 2022
Ionic Modifier Effect on Selectivity of Separation of Deoxyguanosine, Guanine and Guanosine on BIST B+ by SIELC Technologies.
Separation type: Bridge Ion Separation Technology, or BIST™ by SIELC Technologies
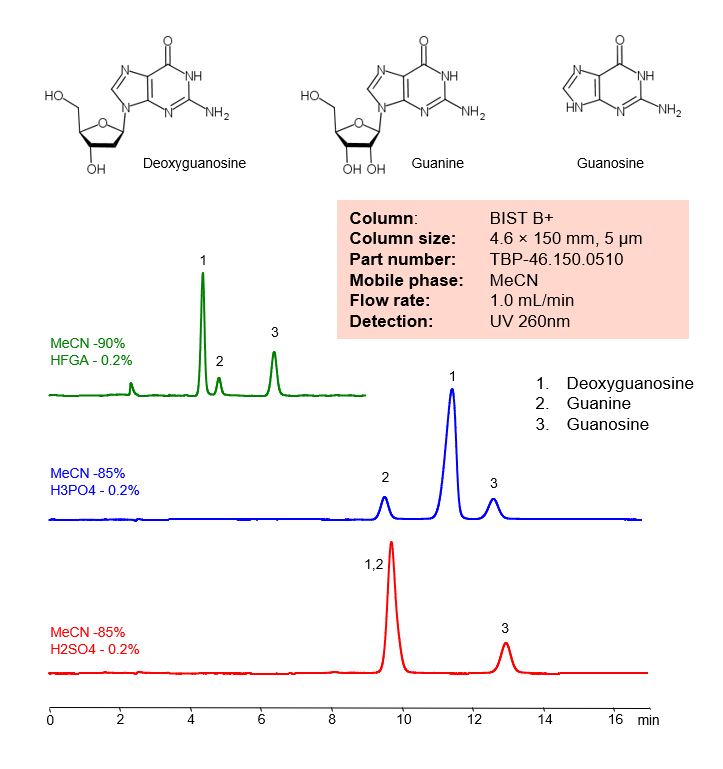
High Performance Liquid Chromatography (HPLC) Method for Analyses of Deoxyguanosine, Guanine and Guanosine
Condition
| Column | BIST B+, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN |
| Buffer | H3PO4, H2SO4, HFGA (Hexafluoroglutaric acid) – 0.2%, |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260 nm |
| Peak Retention Time |
Description
| Class of Compounds | Nucleosides |
| Analyzing Compounds | Deoxyguanosine, Guanine and Guanosine |
Application Column
BIST B+
BIST™ columns offer a unique and effective way to achieve separations that were traditionally challenging or even impossible with other HPLC columns. With the use of a special mobile phase, these ion exchange columns provide very strong retention for analytes with the same charge polarity as the stationary phase, unlocking new chromatography applications. What makes BIST™ columns stand out is their proprietary surface chemistry, which results in superior selectivity, resolution, and sensitivity. These columns offer a simple, efficient solution for a variety of analytical challenges, making them an excellent choice for researchers and analysts across many different fields. To learn more about the technology that powers BIST™ columns and to explore related applications, check out https://BIST.LC.
Select optionsGuanine
Guanosine

HPLC Method for Analysis of Deoxyguanosine, Guanine and Guanosine on BIST B+
December 5, 2022
HPLC Method for Analysis of Deoxyguanosine, Guanine and Guanosine on BIST B+ by SIELC Technologies.
Separation type: Bridge Ion Separation Technology, or BIST™ by SIELC Technologies
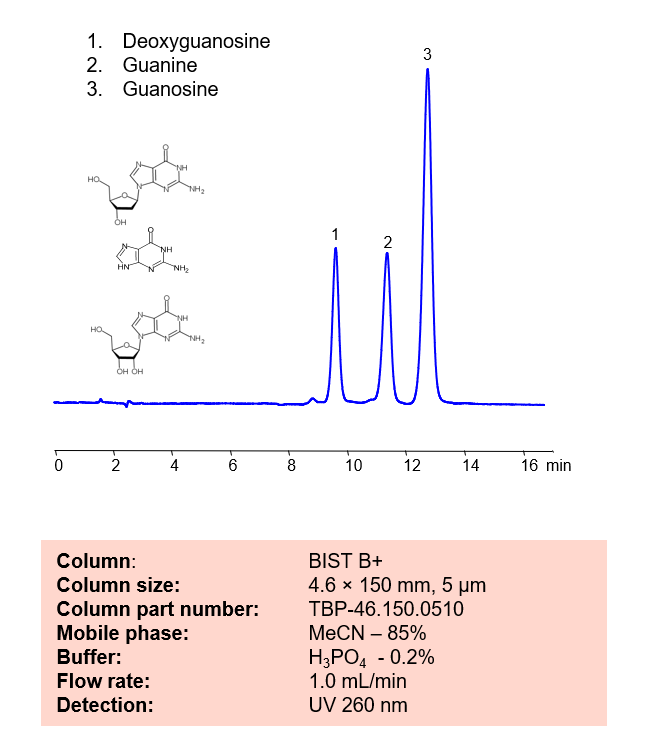
High Performance Liquid Chromatography (HPLC) Method for Analyses of Deoxyguanosine, Guanine and Guanosine
Condition
| Column | BIST B+, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN – 85% |
| Buffer | H3PO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260 nm |
| Peak Retention Time | 9.6, 11.2, 12.8 min |
Description
| Class of Compounds | Nucleosides |
| Analyzing Compounds | Deoxyguanosine, Guanine and Guanosine |
Application Column
BIST B+
BIST™ columns offer a unique and effective way to achieve separations that were traditionally challenging or even impossible with other HPLC columns. With the use of a special mobile phase, these ion exchange columns provide very strong retention for analytes with the same charge polarity as the stationary phase, unlocking new chromatography applications. What makes BIST™ columns stand out is their proprietary surface chemistry, which results in superior selectivity, resolution, and sensitivity. These columns offer a simple, efficient solution for a variety of analytical challenges, making them an excellent choice for researchers and analysts across many different fields. To learn more about the technology that powers BIST™ columns and to explore related applications, check out https://BIST.LC.
Select optionsGuanine
Guanosine

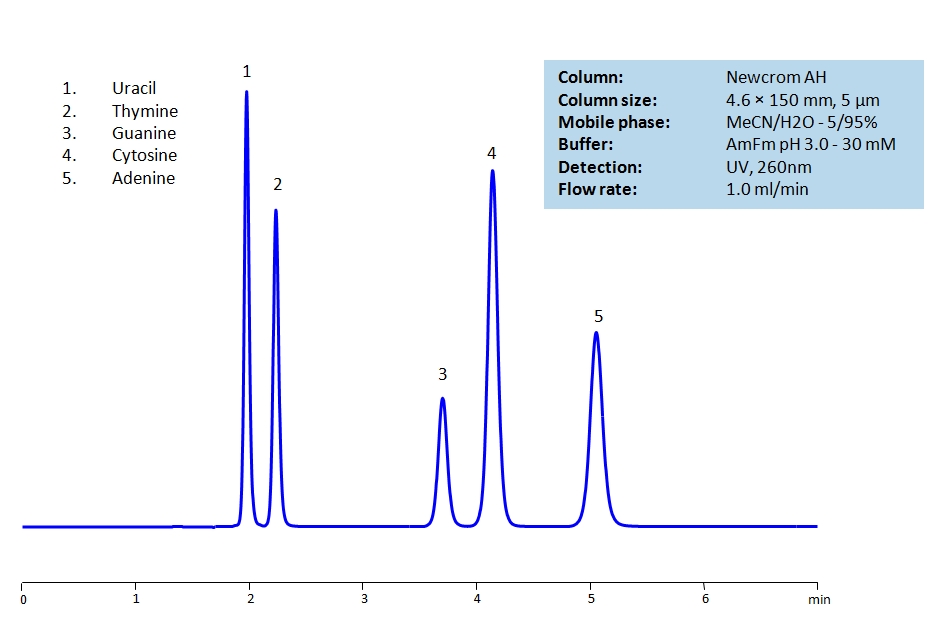
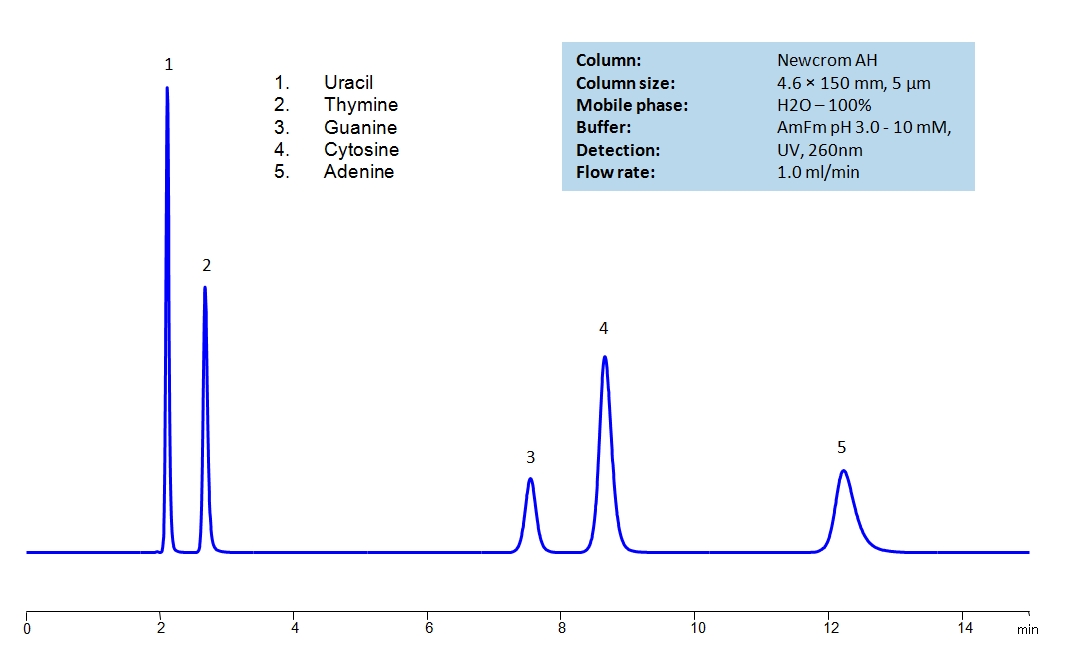
HPLC Separation of Uracil, Thymine, Guanine, Cytosine, Adenine on Newcrom AH
April 14, 2020
Uracil, Thymine, Guanine, Cytosine and Adenine are the nucleobases found in RNA and DNA. The nucleobases are difficult to separate on reverse-phase columns due to their polar, hydrophilic and ionic nature. Using the Newcrom AH mixed-mode column, the nucleobases can be easily separated isocratically using low organic mobile phase (5% acetonitrile) or pure water, if organic mobile phase is undesirable, with ammonium formate buffer, making the method both UV and Mass Spec compatible.
| Column | Newcrom AH, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 5/95% |
| Buffer | AmFm pH 3.0- 30 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260 nm, MS-compatible mobile phase |
| Column | Newcrom AH, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | H2O – 100% |
| Buffer | AmFm pH 3.0- 10 mM |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260 nm, MS-compatible mobile phase |
| Class of Compounds | Hydrophilic, Drug, Xanthine, Nucleic Bases |
| Analyzing Compounds | Uracil, Thymine, Guanine, Cytosine, Adenine |
Application Column
Newcrom AH
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsCytosine
Guanine
Thymine
Uracil

HPLC Application for Separation of Nucleotide Bases Uracil, Thymine, Guanine, Cytosine, Adenine on Primesep 200 Column
December 6, 2007
Nucleotide bases are parts of DNA and RNA. Adenine and guanine are purine-bases; uracil, thymine and cytosine are pyrimidine-bases. In the view of chromatography these compounds are very polar and similar in properties. It is hard to obtain base line HPLC separation on traditional C18 as peaks of nucleotide bases co-elute even at low organic concentration. In this application nucleobases are well retained and separated on Primesep 200 mixed-mode column. Compounds are retained by weak reverse phase and weak ion-exchange mechanisms. This HPLC method can utilize UV, ELSD, and LC/MS detection.
| Column | Primesep 200, 4.6*250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 10/90% |
| Buffer | TFA – 0.2% |
| Flow Rate | 0.5 ml/min, 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Uracil, Thymine, Cytosine, Guanine, Adenine |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsCytosine
Guanine
Purines
Pyrimidines
Uracil

Separation of Nucleic Bases
September 24, 2003
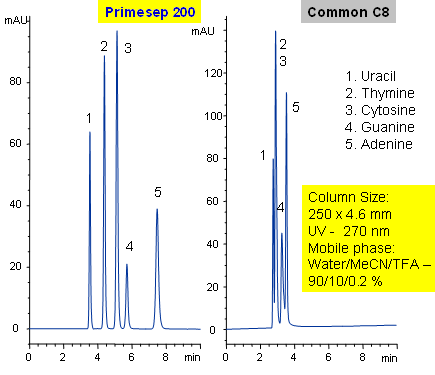
Primesep 200 separates with baseline resolution nucleic bases (uracil, thymine, cytosine, guanine, and adenine) by a combination of cation exchange and reversed phase. Uracil typically does not retain on reversed-phase column and is often used as an unretained void volume marker for C18 and C8 columns. Primesep 200 has an embedded anionic functional group which helps retain polar compounds polar and ion-exchange mechanisms. Excellent peak shape results with a mass spec compatible mobile phase of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) with UV detection at 270 nm.
| Column | Primesep 200, 4.6*250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 10/90% |
| Buffer | TFA – 0.2% |
| Flow Rate | 0.5 ml/min, 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Uracil, Thymine, Cytosine, Guanine, Adenine |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsCytosine
Guanine
Nucleic Bases
Thymine
Uracil