| CAS Number | 71-00-1 |
|---|---|
| Molecular Formula | C6H9N3O2 |
| Molecular Weight | 155.157 |
| InChI Key | HNDVDQJCIGZPNO-YFKPBYRVSA-N |
| LogP | -3.32 |
| Synonyms |
|
Applications:
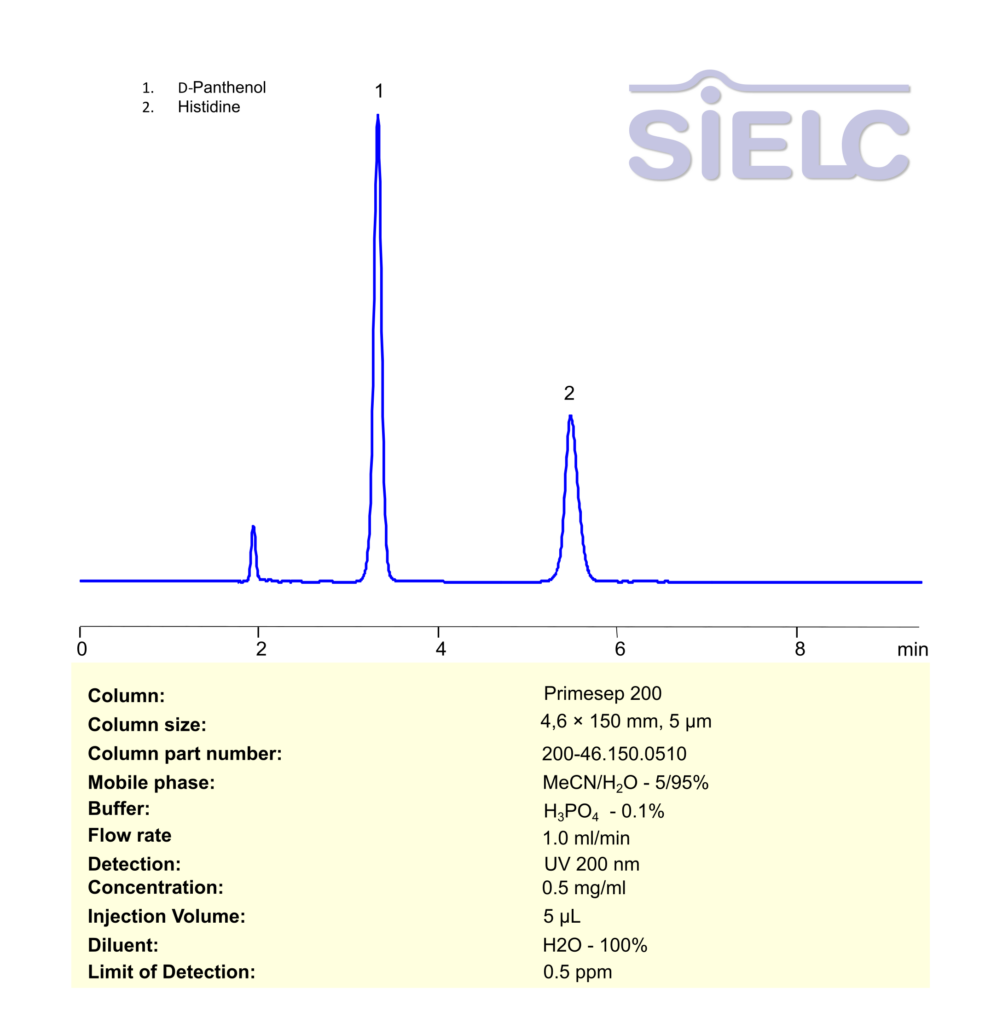
HPLC Method for Analysis of D-Panthenol and Histidine on Primesep 200 Column
November 15, 2024
High Performance Liquid Chromatography (HPLC) Method for Analysis of Panthenol, Histidine on Primesep 200 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Panthenol, Histidine
D-Panthenol and Histidine are two different compounds that are commonly used in skincare, hair care, and other health-related products due to their beneficial properties.
D-Panthenol (Provitamin B5)
Chemical structure: D-Panthenol is the alcohol form of pantothenic acid (Vitamin B5).
Uses: It is widely used in cosmetics and personal care products for its moisturizing and skin-soothing properties.
Benefits:
Hydration: D-Panthenol is known to improve skin and hair moisture retention.
Skin barrier support: It helps in repairing and strengthening the skin’s barrier function, which is essential for maintaining skin health.
Soothing: It has anti-inflammatory properties, making it beneficial for soothing irritation or minor skin damage.
Healing: D-Panthenol supports wound healing and reduces redness or discomfort associated with sunburn, minor wounds, or rashes.
Hair health: It also coats the hair, improving shine, softness, and strength while reducing the appearance of split ends.
Histidine
Chemical structure: Histidine is an essential amino acid involved in several metabolic processes and is a building block for proteins.
Uses: In the skincare and health sectors, histidine is used for its antioxidant and soothing properties.
Benefits:
Anti-inflammatory: Histidine can help reduce inflammation in the skin, which can be helpful in conditions like eczema or acne.
Antioxidant: It helps protect the skin from oxidative stress caused by free radicals, which can lead to premature aging and damage.
pH regulation: Histidine can help balance the skin’s pH levels, promoting a healthy skin environment.
Skin hydration: By supporting the skin’s natural processes, histidine aids in moisture retention and overall skin smoothness.
Barrier function: It supports the repair and maintenance of the skin’s protective barrier, helping it defend against external irritants.
Both D-Panthenol and Histidine are valuable ingredients in formulations aimed at improving skin hydration, soothing irritation, and maintaining a healthy barrier.
Panthenol, Histidine can be retained, separated and analyzed using a Primesep 200 mixed-mode stationary phase column. The analysis employs an isocratic method with a simple mobile phase comprising water, acetonitrile (MeCN), and phosphoric acid as a buffer. This method allows for detection using UV 200 nm.
| Column | Primesep 200, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 5% |
| Buffer | H3PO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 |
| Sample | 0.5 mg/mL |
| Diluent | H2O- 100% |
| LOD* | 0.5 ppm |
Application Column
Primesep 200
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Panthenol

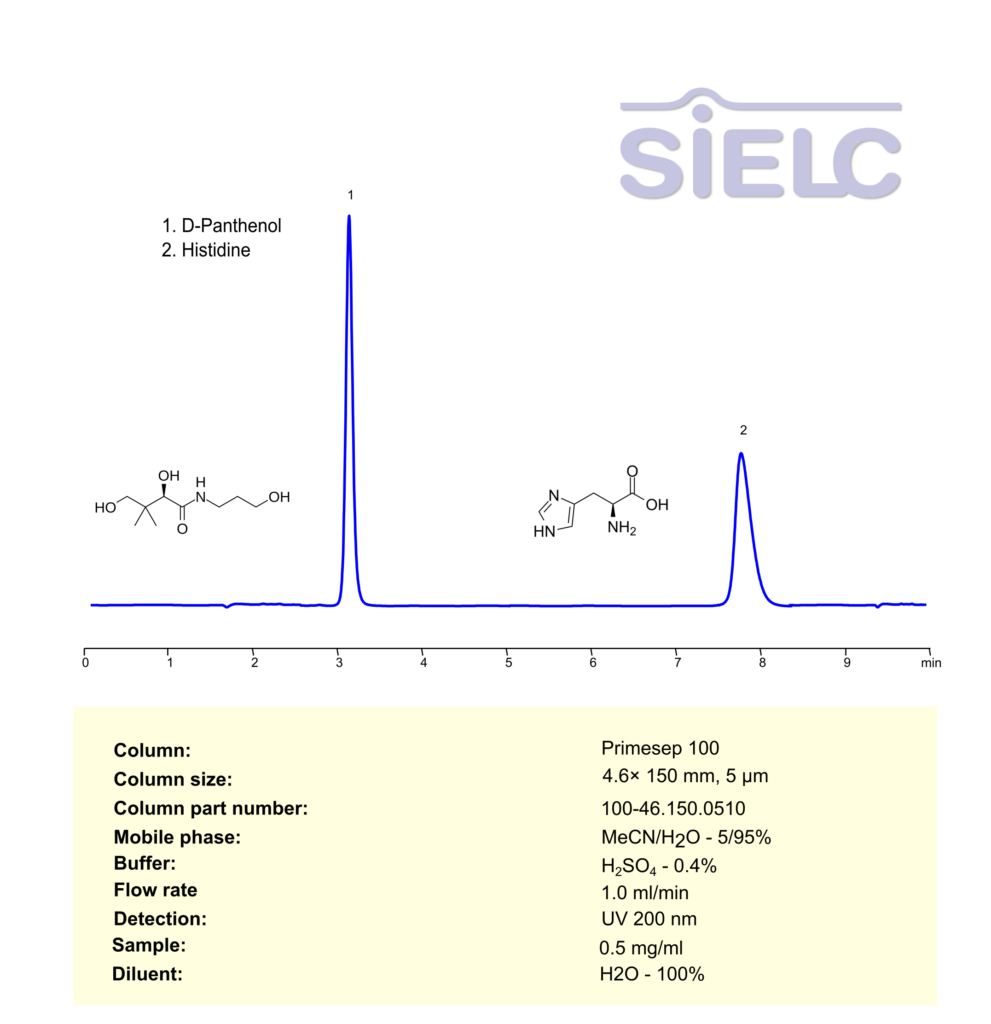
HPLC Method for Analysis of D-Panthenol and Histidine on Primesep 100 Column
October 22, 2024
High Performance Liquid Chromatography (HPLC) Method for Analysis of Panthenol, Histidine on Primesep 100 by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Panthenol, Histidine
D-Panthenol and Histidine are two different compounds that are commonly used in skincare, hair care, and other health-related products due to their beneficial properties.
D-Panthenol (Provitamin B5)
Chemical structure: D-Panthenol is the alcohol form of pantothenic acid (Vitamin B5).
Uses: It is widely used in cosmetics and personal care products for its moisturizing and skin-soothing properties.
Benefits:
Hydration: D-Panthenol is known to improve skin and hair moisture retention.
Skin barrier support: It helps in repairing and strengthening the skin’s barrier function, which is essential for maintaining skin health.
Soothing: It has anti-inflammatory properties, making it beneficial for soothing irritation or minor skin damage.
Healing: D-Panthenol supports wound healing and reduces redness or discomfort associated with sunburn, minor wounds, or rashes.
Hair health: It also coats the hair, improving shine, softness, and strength while reducing the appearance of split ends.
Histidine
Chemical structure: Histidine is an essential amino acid involved in several metabolic processes and is a building block for proteins.
Uses: In the skincare and health sectors, histidine is used for its antioxidant and soothing properties.
Benefits:
Anti-inflammatory: Histidine can help reduce inflammation in the skin, which can be helpful in conditions like eczema or acne.
Antioxidant: It helps protect the skin from oxidative stress caused by free radicals, which can lead to premature aging and damage.
pH regulation: Histidine can help balance the skin’s pH levels, promoting a healthy skin environment.
Skin hydration: By supporting the skin’s natural processes, histidine aids in moisture retention and overall skin smoothness.
Barrier function: It supports the repair and maintenance of the skin’s protective barrier, helping it defend against external irritants.
Both D-Panthenol and Histidine are valuable ingredients in formulations aimed at improving skin hydration, soothing irritation, and maintaining a healthy barrier.
Panthenol, Histidine can be retained, separated and analyzed using a Primesep 100 mixed-mode stationary phase column. The analysis employs an isocratic method with a simple mobile phase comprising water, acetonitrile (MeCN), and sulfuric acid as a buffer. This method allows for detection using UV 200 nm.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 5% |
| Buffer | H2SO4 – 0.4% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 200 |
| Sample | 0.5 mg/mL |
| Diluent | H2O- 100% |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Panthenol

UV-Vis Spectrum of Histidine
July 8, 2024
Access the UV-Vis Spectrum SIELC Library
If you are looking for optimized HPLC method to analyze Histidine check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.

HPLC Method for Separation of Histidine, Histidine Methyl Ester and Histidine lauryl Ester on BIST B+ Column
March 23, 2023
HPLC Method for Separation of Histidine, Histidine Methyl Ester, and Histidine Lauryl Ester on BIST B+ by SIELC Technologies
Separation type: Bridge Ion Separation Technology, or BIST™ by SIELC Technologies
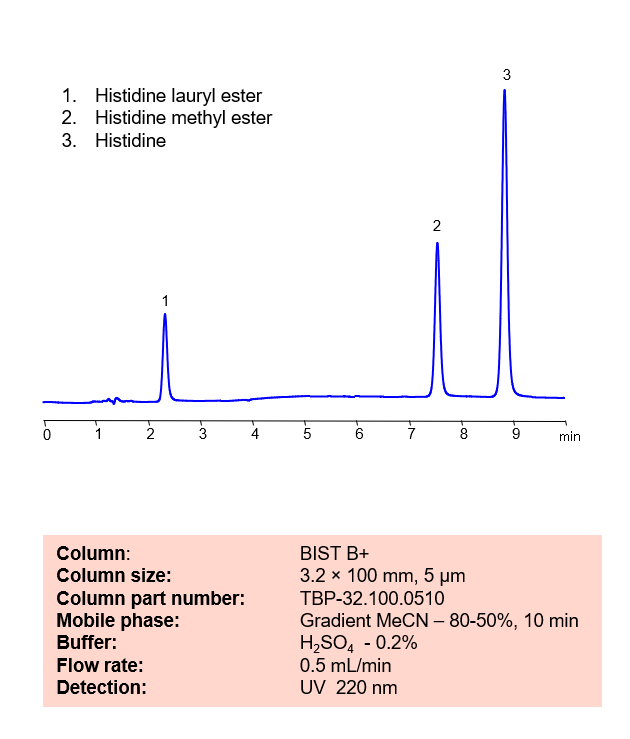
Histidine is a naturally occurring essential amino acid that the body uses to repair damaged tissue and generate new blood cells. Histidine Methyl Ester is a histidine decarboxylase inhibitor and can be used to synthesize other biological compounds. Histidine Lauryl Ester is another derivative of Histidine. Using SIELC’s newly introduced BIST™ method, Histidine and its derivatives can be retained and separated on a positively charged, anion-exchange BIST™ B+ column. There are two keys to this retention method: 1) a multi-charged, negative buffer, such as Sulfuric acid (H2SO4), which acts as a bridge, linking the positively charged dipeptide to the positively charged column surface and 2) a mobile phase consisting mostly of organic solvent (such as MeCN) to minimize the formation of a solvation layer around the charged analytes. Using this new and unique analysis method, Histidine and its derivatives can be UV detected at 220 nm.
High Performance Liquid Chromatography (HPLC) Method for Analysis of Histidine, Histidine Methyl Ester and Histidine lauryl Ester
Condition
| Column | BIST B+, 3.2 x 100 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | Gradient MeCN – 80 – 50%, 10 min |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 0.5 ml/min |
| Detection | UV 220 nm |
| Peak Retention Time | 2.2 min, 7.5 min, 8.9 min |
Description
| Class of Compounds | Amino acid |
| Analyzing Compounds | Histidine methyl ester, Histidine |
Application Column
BIST B+
Column Diameter: 3.2 mm
Column Length: 100 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Histidine methyl ester

HPLC Method for Analysis of Arginine, Lysine and Histidine Amino Acids on BIST B+
November 30, 2022
HPLC Method for Analysis of Arginine, Lysine, Histidine on BIST B+ by SIELC Technologies.
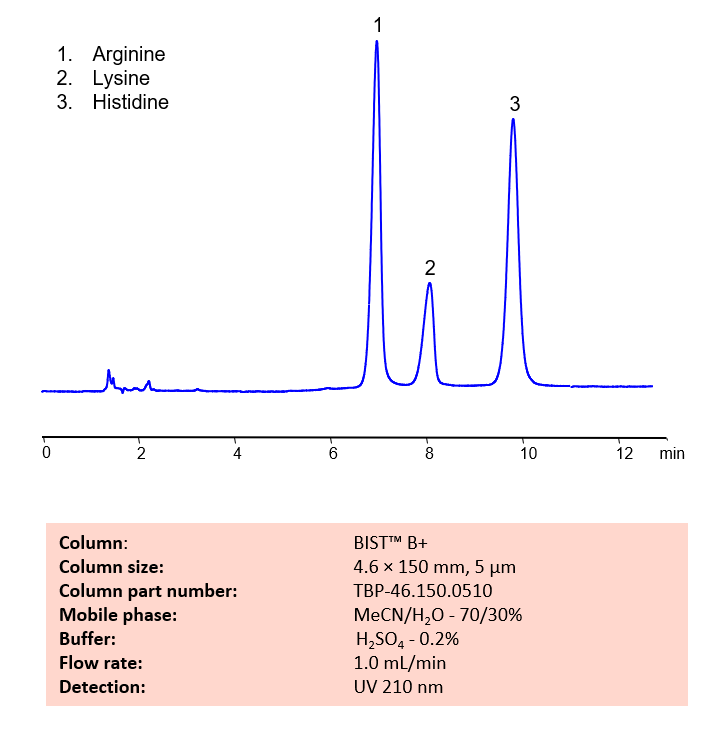
Arginine is a semiessential amino acid with the chemical formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. It was originally isolated from yellow lupin seedlings in 1886 by Ernst Schulze and Enst Steiger. Now, commercially, it is obtained through fermentation. Arginine can be typically found in meat, poultry, and fish. You can find detailed UV spectra of Arginine and information about its various lambda maxima by visiting the following link.
Lysine is an essential amino acid with the chemical formula C6H14N2O2. It’s primary role is in proteinogenesis, but it also plays a part in uptake of essential mineral nutrients, production of carnitine, and histone modification. Lysine is known in pop culture thanks to Jurassic Park, in which dinosaurs were genetically altered to be unable to produce lysine. While in the story, this made the dinosaurs be unable to survive in the wild due to needing the lysine supplements provided by the staff at Jurassic Park, no real animal can produce lysine naturally. Lysine is another essential amino acid typically found in meat, and also cereal grains. You can find detailed UV spectra of Lysine and information about its various lambda maxima by visiting the following link.
Histidine is an essential amino acid with the chemical formula C9H9N3O2. It cannot be produced within the body and must be obtained through consuming it. It s fundamental for repair of damaged tissue, growth of muscles, and making of blood cells through biosynthesis of protein. Outside of protein, it also has the unique property of being able to act as a buffer to help maintain stable pH levels in the body. Sources of it include meat, fish, dairy products, beans, and nuts. Histidine, is also typtically found in meat, poultry, and also cereal grains (like rice, wheat, and rye). You can find detailed UV spectra of Lysine and information about its various lambda maxima by visiting the following link.
Basic amino acids, like Arginine, Lysine, Histidine can be difficult to separate and retain in typical reverse-phase chromatography. Using SIELC’s newly introduced BIST™ method, these three essential amino acids, which protonate in water, can be retained on a positively-charged anion-exchange BIST B+ column. There are two keys to this retention method: 1) a multi-charged, negative buffer, such as Sulfuric acid (H2SO4), which acts as a bridge, linking the positively-charged amino acid analytes to the positively-charged column surface and 2) a mobile phase consisting mostly of organic solvent (such as MeCN) to minimize the formation of a solvation layer around the charged analytes. Using this new and unique analysis method, Arginine, Lysine, and Histidine can be retained and UV detected at 210 nm.
Condition
| Column | BIST B+, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 70% |
| Buffer | H2SO4 – 0.2% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 210 nm |
| Peak Retention Time | 6.2, 8.0, 9.9 min |
Description
Application Column
BIST B+
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Histidine
Lysine

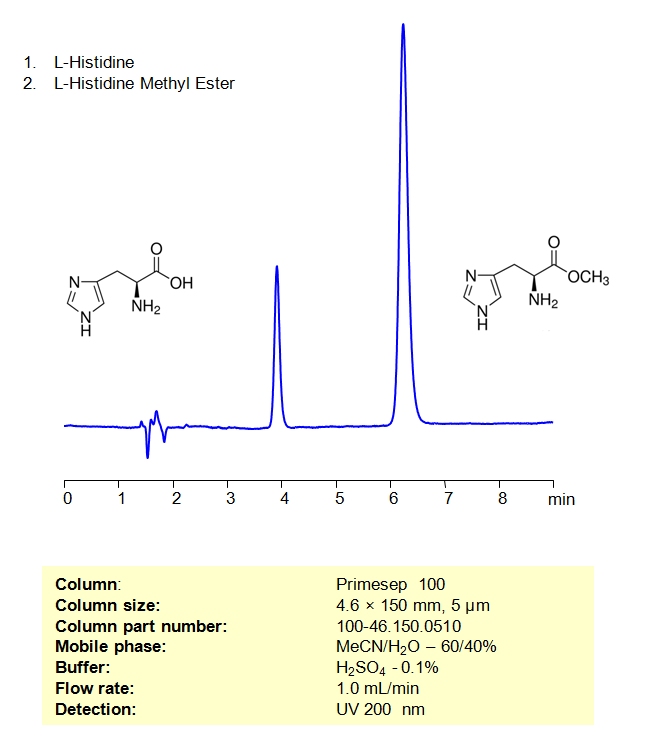
HPLC Method For Analysis Of Histidine and Histidine Methyl Ester Dihydrochloride on Primesep 100 Column
January 27, 2022
Separation type: Liquid Chromatography Mixed-mode
High Performance Liquid Chromatography (HPLC) Method for Analysis of Histidine and Histidine Methyl Ester Dihydrochloride
Histidine and Histidine Methyl Ester Dyhydrochloride are related compounds with interesting uses. Histidine is used in biosynthesis to create proteins, while histidine methyl ester dihydrochloride (a derivative of histidine) is used to create various other compounds.
This amino acid and its derivative can be detected in the low UV regime. Using a Primesep 100 mixed-mode column and a mobile phase consisting of water and acetonitrile (MeCN) with a sulfuric acid (H2SO4) buffer, Histidine and Histidine Methyl Ester Dyhydrochloride can be retained, separated, and analyzed. This analysis method can be UV detected at 200 nm.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 60/40% |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds |
Amino acid |
| Analyzing Compounds | Histidine, Histidine Methyl Ester |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsHistidine methyl ester
L-Histidine hydrochloride monohydrate

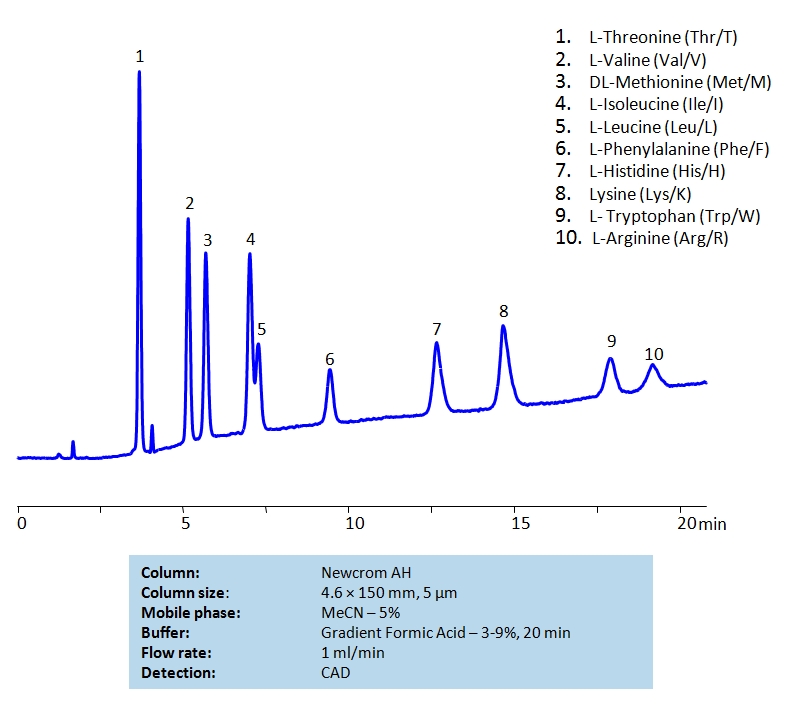
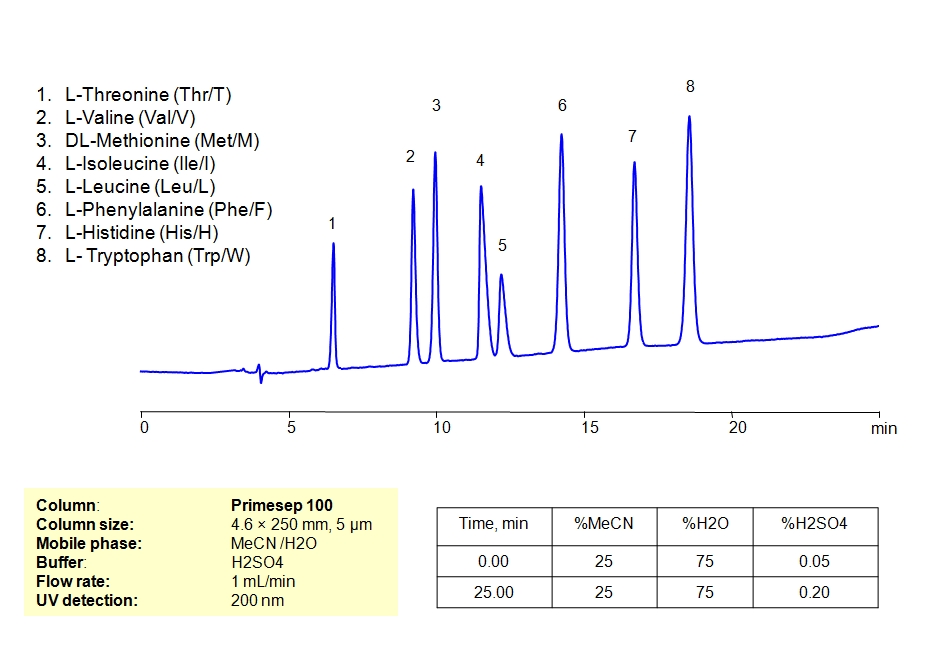
HPLC Separation of Mixture of Nine Essential Amino acids and Arginine on Newcrom AH Column
September 25, 2020
HPLC Method for L-Threonine, Valine, Methionine, Isoleucine, Leucine, Phenylalanine, Histidine, Tryptophan, Lysine, Arginine on Newcrom AH by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of L-Threonine, Valine, Methionine, Isoleucine, Leucine, Phenylalanine, Histidine, Tryptophan, Lysine, Arginine.
Essential amino acids cannot be made by the body. As a result, they must come from food.
The 9 essential amino acids are: histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, and valine.
L-Threonine is an essential amino acid with the chemical formula C4H9NO3. It cannot be produced within the body and must be obtained through consuming it. It’s found in many protein-rich foods, including but not limited to eggs, meat, dairy, legumes, and seeds. It is necessary in the body as a building block of protein like collagen and elastin. The two proteins are crucial for skin, hair, and connective issue.
L-Valine is an essential amino acid with the chemical formula C5H11NO2. It cannot be produced within the body and must be obtained through consuming it. It’s found in foods including but not limited to nuts, legumes, whole grains, and seeds. It is especially beneficial for athletes. It is important for muscle repair, growth, and energy regulation.
DL-Methionine is an essential amino acid with the chemical formula C5H11NO2S. It cannot be produced within the body and must be obtained through consuming it. It is required for protein synthesis. It also helps build and repair tissue including, but not limited to, skin, hair, muscles, and nails. In a veterinary context, DL-Methionine is used to address bladder issues in dogs.
L-Isoleucine is an essential amino acid with the chemical formula C6H13NO2. It cannot be produced within the body and must be obtained through consuming it. It is a building block of protein that are essential for muscle growth, repair, and other bodily functions. It also helps regulate blood sugar levels and supports the immune system. It is found in foods like meat, fish, eggs, dairy, beans, lentils, nuts, and seeds.
L-Leucine is an essential amino acid with the chemical formula C6H13NO2. It cannot be produced within the body and must be obtained through consuming it. It stimulates production of protein that are essential for muscle building and repair. Meats are the easiest way to get L-Leucine in significant amounts.
L-Phenylalanine is an essential amino acid with the chemical formula C6H9NO2. It cannot be produced within the body and must be obtained through consuming it. It is typically found in high protein foods such as meat, eggs, and fish. Outside of being important for creation of protein, it is also used in treatment for skin disorders and depression.
L-Histidine is an essential amino acid with the chemical formula C9H11N3O2. It cannot be produced within the body and must be obtained through consuming it. It s fundamental for repair of damaged tissue, growth of muscles, and making of blood cells. Outside of protein, it also has the unique property of being able to act as a buffer to help maintain stable pH levels in the body. Sources of it include meat, fish, dairy products, beans, and nuts.
L-Tryptophan is an essential amino acid with the chemical formula C11H12N2O2. It cannot be produced within the body and must be obtained through consuming it. Like the other essential proteins, it is a building block for protein and muscle tissue, but it is also converted in the body into serotonin, which affects mood. L-Tryptophan is also used in treatments for severe PMS symptoms, depression, and insomnia. It is naturally found in red meat, poultry eggs, and dairy.
Lysine is an essential amino acid used in the synthesis of proteins. In biological conditions, it is a basic, charged molecule.
L-Arginine is an essential amino acid used in the synthesis of proteins.
L-Threonine, Valine, Methionine, Isoleucine, Leucine, Phenylalanine, Histidine, Tryptophan, Lysine, Arginine can be retained and analyzed using the Newcrom AH stationary phase column. The analysis utilizes a gradient method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a Formic Acid buffer. Detection is performed using CAD.
| Column | Newcrom AH, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN – 5% |
| Buffer | Gradient Formic Acid – 3-9%, 20 min |
| Flow Rate | 1.0 ml/min |
| Detection | CAD |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | L-Threonine, Valine, Methionine, Isoleucine, Leucine, Phenylalanine, Histidine, Tryptophan, Lysine, Arginine |
Application Column
Newcrom AH
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Histidine
Isoleucine
L-Threonine
Leucine
Lysine
Methionine
Phenylalanine
Tryptophan
Valine

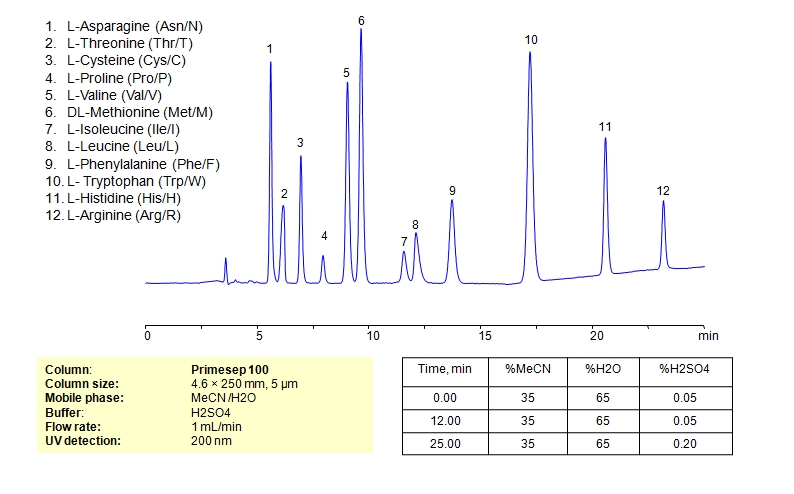
HPLC Separation of Mixture of 12 Amino Acids on Primesep 100 Column
March 11, 2019
HPLC Method for Asparagine, L-Cysteine, Cysteine, Proline, Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, D-Leucine, Phenylalanine, Tryptophan, Histidine, Arginine, Amino Acids, Leucine, L-Threonine on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Asparagine, L-Cysteine, Cysteine, Proline, Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, D-Leucine, Phenylalanine, Tryptophan, Histidine, Arginine, Amino Acids, Leucine, L-Threonine.
Amino acids are the building blocks of proteins. Based on their dietary requirement, they are classified into essential and non-essential amino acids. Essential amino acids cannot be synthesized by the human body in sufficient quantities and must be obtained from the diet. Non-essential amino acids, on the other hand, can be synthesized by the body and are not dependent on dietary intake.
It’s worth noting that while these amino acids are considered “non-essential” for adults under normal circumstances because the body can synthesize them, there are situations where some may become “conditionally essential.” This means that under certain conditions like illness, stress, or trauma, the body might not produce them in sufficient quantities, and dietary intake becomes necessary. Arginine, for instance, is considered conditionally essential, especially during periods of rapid growth, illness, or trauma.
Amino acids can be retained, separeted and analyzed on a Primesep 100 mixed-mode stationary phase column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a sulfuric acid (H2SO4) as a buffer. This analysis method can be detected in the UV regime at 200 nm.
| Column | Primesep 100, 4.6 x 250 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 35/65% |
| Buffer | H2SO4 0.05% 12 min hold, gradient 0.05-0.20, 13 min |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Asparagine, L-Cysteine, Cysteine, Proline, Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, D-Leucine, Phenylalanine, Tryptophan, Histidine, Arginine, Amino Acids, Leucine, L-Threonine |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 250 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Arginine
Asparagine
Cysteine
D-Isoleucine
D-Leucine
D-Valine
DL-Isoleucine
Histidine
Isoleucine
L-Cysteine
L-Methionine
L-Threonine
Leucine
Methionine
Phenylalanine
Proline
Tryptophan
Valine

HPLC Separation of Mixture of Essential Amino Acids on Primesep 100 Column
March 11, 2019
HPLC Method for Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, L-Isoleucine, D-Leucine, Phenylalanine, Histidine, L-Histidine hydrochloride monohydrate, Tryptophan, Amino Acids, L-Threonine on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, L-Isoleucine, D-Leucine, Phenylalanine, Histidine, L-Histidine hydrochloride monohydrate, Tryptophan, Amino Acids, L-Threonine.
L-Threonine is an essential amino acid with the chemical formula C4H9NO3. It cannot be produced within the body and must be obtained through consuming it. It’s found in many protein-rich foods, including but not limited to eggs, meat, dairy, legumes, and seeds. It is necessary in the body as a building block of protein like collagen and elastin. The two proteins are crucial for skin, hair, and connective issue.
L-Valine is an essential amino acid with the chemical formula C5H11NO2. It cannot be produced within the body and must be obtained through consuming it. It’s found in foods including but not limited to nuts, legumes, whole grains, and seeds. It is especially beneficial for athletes. It is important for muscle repair, growth, and energy regulation.
DL-Methionine is an essential amino acid with the chemical formula C5H11NO2S. It cannot be produced within the body and must be obtained through consuming it. It is required for protein synthesis. It also helps build and repair tissue including, but not limited to, skin, hair, muscles, and nails. In a veterinary context, DL-Methionine is used to address bladder issues in dogs.
L-Isoleucine is an essential amino acid with the chemical formula C6H13NO2. It cannot be produced within the body and must be obtained through consuming it. It is a building block of protein that are essential for muscle growth, repair, and other bodily functions. It also helps regulate blood sugar levels and supports the immune system. It is found in foods like meat, fish, eggs, dairy, beans, lentils, nuts, and seeds.
L-Leucine is an essential amino acid with the chemical formula C6H13NO2. It cannot be produced within the body and must be obtained through consuming it. It stimulates production of protein that are essential for muscle building and repair. Meats are the easiest way to get L-Leucine in significant amounts.
L-Phenylalanine is an essential amino acid with the chemical formula C6H9NO2. It cannot be produced within the body and must be obtained through consuming it. It is typically found in high protein foods such as meat, eggs, and fish. Outside of being important for creation of protein, it is also used in treatment for skin disorders and depression.
L-Histidine is an essential amino acid with the chemical formula C9H11N3O2. It cannot be produced within the body and must be obtained through consuming it. It s fundamental for repair of damaged tissue, growth of muscles, and making of blood cells. Outside of protein, it also has the unique property of being able to act as a buffer to help maintain stable pH levels in the body. Sources of it include meat, fish, dairy products, beans, and nuts.
L-Tryptophan is an essential amino acid with the chemical formula C11H12N2O2. It cannot be produced within the body and must be obtained through consuming it. Like the other essential proteins, it is a building block for protein and muscle tissue, but it is also converted in the body into serotonin, which affects mood. L-Tryptophan is also used in treatments for severe PMS symptoms, depression, and insomnia. It is naturally found in red meat, poultry eggs, and dairy.
Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, L-Isoleucine, D-Leucine, Phenylalanine, Histidine, L-Histidine hydrochloride monohydrate, Tryptophan, Amino Acids, L-Threonine can be retained and analyzed using the Primesep 100 stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water and acetonitrile (MeCN) with a sulfuric acid buffer. Detection is performed using UV.
| Column | Primesep 100, 4.6 x 250 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 25/75% |
| Buffer | Gradient H2SO4 0.05-0.2% 25 min |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Valine, D-Valine, Methionine, L-Methionine, Isoleucine, D-Isoleucine, DL-Isoleucine, L-Isoleucine, D-Leucine, Phenylalanine, Histidine, L-Histidine hydrochloride monohydrate, Tryptophan, Amino Acids, L-Threonine |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 250 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
D-Isoleucine
D-Leucine
D-Valine
DL-Isoleucine
Histidine
Isoleucine
L-Histidine hydrochloride monohydrate
L-Isoleucine
L-Methionine
L-Threonine
Methionine
Phenylalanine
Tryptophan
Valine

HPLC Separation of Amino Acids in Zero Organic Mode on Primesep 200 column
July 8, 2011
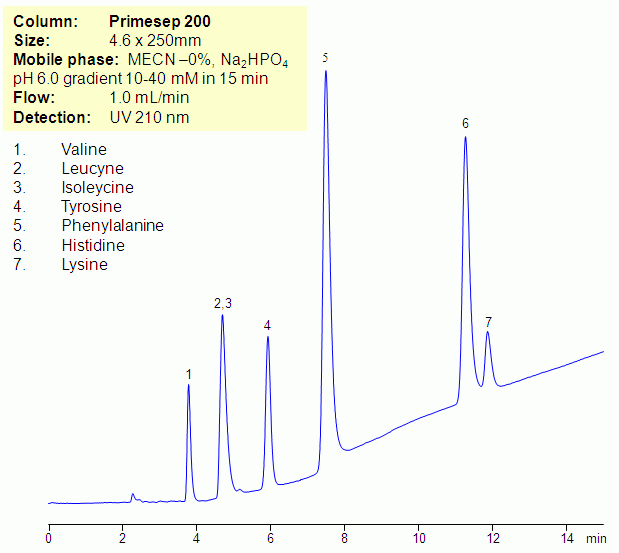
Essential and non-essential amino acids can be retained and separated in zero-organic mode on Primesep mixed-mode HPLC columns. Zero-organic mode is required to monitor isotopes of carbon. Amino acids are retained by combination of reversed-phase and cation-exchange mechanisms. At lower pH, some of the amino acids are more hydrophobic. Buffer pH will affect ionization state of amino acids, and at higher pH (above 2.5), the amino acids will be less hydrophobic and retentive in zero-organic mode. Amino acids can be monitored by low UV. Method can be used in archeological research for analysis of various molecules where presence of organic component of the mobile phase interferes with analysis.
| Column | Primesep 200, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | Na2HPO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Valine, Leucyne, Isoleycine, Tyrosine, Phenylalanine, Histidine, Lysine |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsIsoleucine
Leucine
Lysine
Phenylalanine
Tyrosine
Valine

HPLC Analysis of Polysorbate in Mixture with Amino Acids and Sugar
July 16, 2009
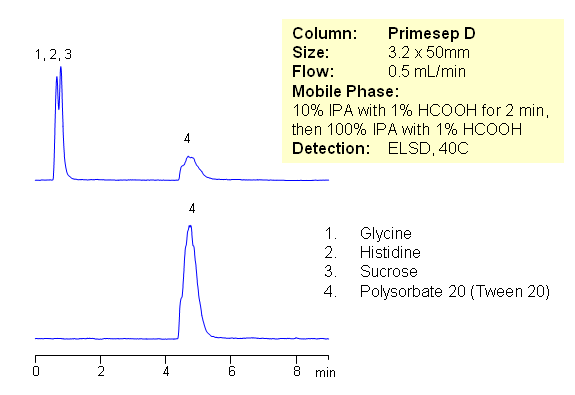
Polysorbates are a class of emulsifiers used in some pharmaceuticals and food preparation. They are often used in cosmetics to solubilize essential oils into water-based products. Polysorbates are oily liquids derived from PEG-ylated sorbitan (a derivative of sorbitol) esterified with fatty acids. Surfactants that are esters of plain (non-PEG-ylated) sorbitan with fatty acids are usually referred to by the name Span. It is often required to quantitate Polysorbate (Polysorbate/Tween 20, Polysorbate/Tween 40, Polysorbate 60/Tween 60 and Polysorbate 80) by HPLC in various formulations. Polysorbates exist in form of oligomers.
| Column | Primesep D, 3.2×50 mm, 5 µm, 100A |
| Mobile Phase | IPA |
| Buffer | Formic Acid |
| Flow Rate | 0.5 ml/min |
| Detection | ELSD |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Glycine, Histidine, Sucrose, Polysorbate 20 |
Application Column
Primesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsGlycine
Histidine
Polysorbate
Polysorbate 20
Polysorbate 80
Sucrose
Tween 20
Tween 80

HPLC-ELSD Method for Analysis of Amino Acids on Primesep 100 Column
September 18, 2006
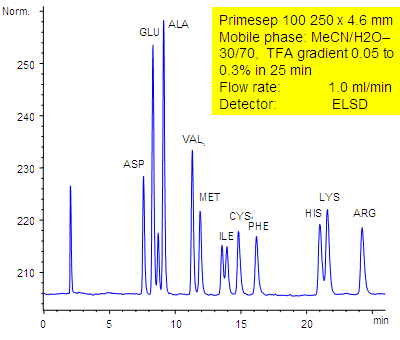
11 underivatized amino acids (aspartic acid, glutamic acid, alanine, valine, methionine, isoleucine, cysteine, phenylalanine, histidine, lysine, and arginine) are separated by a Primesep 100 HPLC column by reversed-phase and ion-exchange mechanisms with LC/MS compatible conditions without the use of ion-pair reagents. The HPLC separation uses a TFA (trifluoroacetic acid) gradient in a mobile phase of water acetonitrile (MeCN, ACN with evaporative light scattering detection (ELSD).
| Column | Primesep 100, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | TFA , gradient 0.05-0.3 % , 25 min |
| Flow Rate | 1.0 ml/min |
| Detection | ELSD |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Aspartic acid, Glutamic acid, Alanine, Valine, Methionine, Isoleucine, Cysteine, Phenylalanine, Histidine, Lysine, Arginine |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsAmino Acids
Arginine
Aspartic Acid
Cysteine
Glutamic Acid
Histidine
Isoleucine
Lysine
Methionine
Phenylalanine
Valine

HPLC Separation of Creatine, Creatinine and Histidine with MS Conditions
January 13, 2005
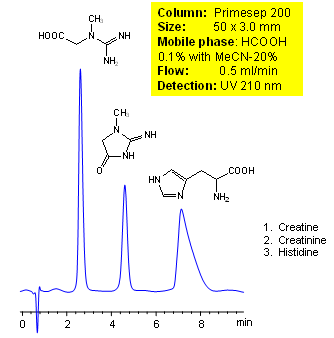
Creatine, creatinine, and histidine are all important amino acids in the human body. All of these can be separated by liquid chromatography. The mobile phase contains acetonitrile (MeCN), water, and phormic acid. Smaller 3 µm particle columns are available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation of impurities in preparative separation. It also suitable for pharmacokinetics.
| Column | Primesep 200, 3.0×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 20/80% |
| Buffer | Formic Acid – 0.1% |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Creatine, Creatinine, Histidine |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsCreatinine
Histidine

HPLC Separation of Creatine, Creatinine and Histidine
January 13, 2005
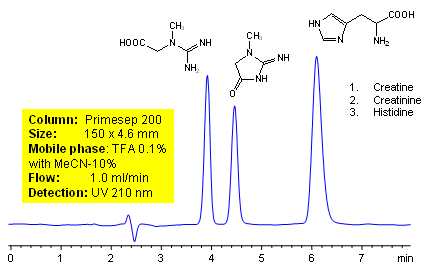
Creatine is produced by the body and plays a major role in recycling ATP. It also stabilizes pH in certain tissues. Creatine can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains acetonitrile (MeCN), water, and TFA. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particle columns are available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation of impurities in preparative separation. It also suitable for pharmacokinetics.
| Column | Primesep 200, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 10/90% |
| Buffer | TFA -0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Creatine, Creatinine, Histidine |
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsCreatinine
Histidine