| CAS Number | 26159-34-2 |
|---|---|
| Molecular Formula | C14H13NaO3 |
| Molecular Weight | 252.245 |
| InChI Key | CDBRNDSHEYLDJV-FVGYRXGTSA-M |
| LogP | 2.22 |
| Synonyms |
|
Applications:
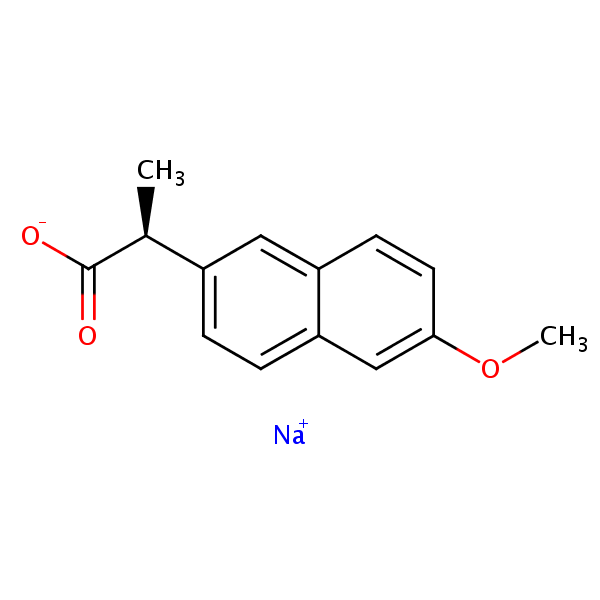
HPLC Determination of Naproxen on Primesep 100 Column
July 30, 2020
Application Notes: Naproxen is a common NSAID used for pain relief and fever reducer.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 40/60% |
| Buffer | H2SO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Hydrophobic, Ionizable |
| Analyzing Compounds | Naproxen |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsNaproxen Sodium

USP Methods for the Analysis of Naproxen Using Legacy L1 Column
June 21, 2012
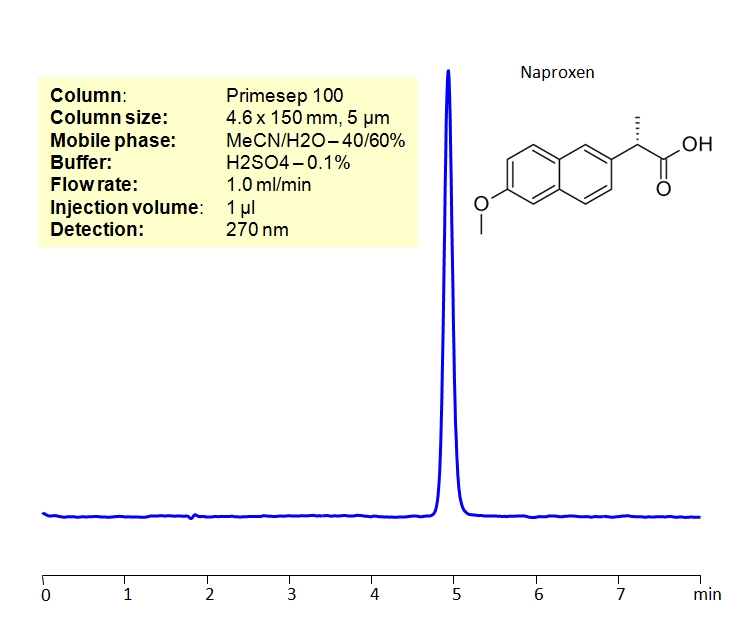
Application Notes: Naproxen is a common NSAID used for pain relief and fever reducer. According to the USP methods, naproxen contains no less than 98% and no more than 101.5 percent naproxen calculated on a dried basis. The USP HPLC method for the separation of naproxen was developed on Legacy L1 column according to the US Pharmacopeia methodology. L1 classification is assigned to reversed-phase HPLC column containing C18 ligand. Support for the material is spherical silica gel with particles size 3-10 um and pore size of 100-120A. Resolution between critical pairs corresponds to rules and specifications of USP.
Application Columns: Legacy L1 C18 HPLC column
Application compounds: naproxen and benzophenone
Mobile phase: water/MeCN/Glacial Acetic acid (49/50/1)
Detection technique: UV
Reference: USP35: NF30
| Column | Legacy L1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | H2O/MeCN/Glacial Acetic acid (49/50/1) |
| Buffer | Glacial Acetic acid |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 254 nm |
| Class of Compounds |
Drug, Hydrophobic, Ionizable |
| Analyzing Compounds | Naproxen, Benzophenone |
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select options
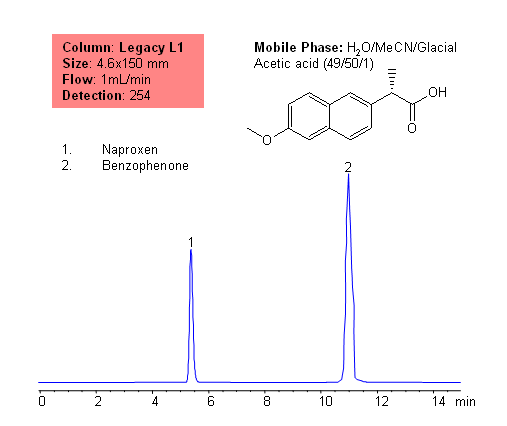
HPLC Separation of Acidic Drugs and Counter-Ions on Obelisc R Column
October 4, 2010
Mixed-mode columns allow to quantitate drugs and their counter-ions in one run. Hydrophobic acidic drug and hydrophilic inorganic counter-ions are separated and quantified on an Obelisc R HPLC trimodal column. This column can be used to quantify hydrophilic basic and hydrophilic acidic drugs, hydrophobic basic and hydrophobic acidic drugs, and corresponding organic and inorganic counter-ions. Various detection techniques can be applied to obtain reliable and robust method. Mixed-mode chromatography allows to retain ionizable compounds without ion-pairing reagent. This HPLC method can be adopted as universal and robust approach for analysis basic and acidic drugs and their counter-ions. Method is compatible with various detection techniques – ELSD, UV, CAD, and LC/MS.
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsIbuprofen Sodium
Naproxen Sodium
UV Detection