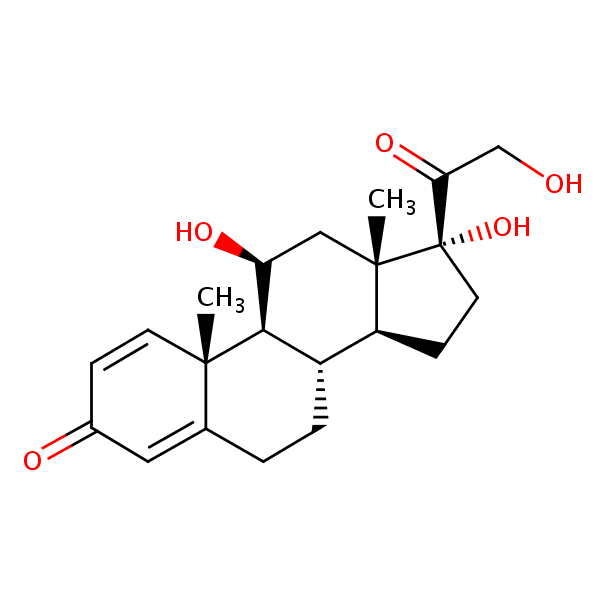
| CAS Number | 50-24-8 |
|---|---|
| Molecular Formula | C21H28O5 |
| Molecular Weight | 360.451 |
| InChI Key | OIGNJSKKLXVSLS-VWUMJDOOSA-N |
| LogP | 1.62 |
| Synonyms |
|
Applications:
HPLC Method for Separating Corticosteroids such as Prednisone, Prednisolone and Methylprednisolone on Primesep B Column
January 29, 2024
HPLC Method for Prednisone, Prednisolone, Methylprednisolone on Primesep B by SIELC Technologies
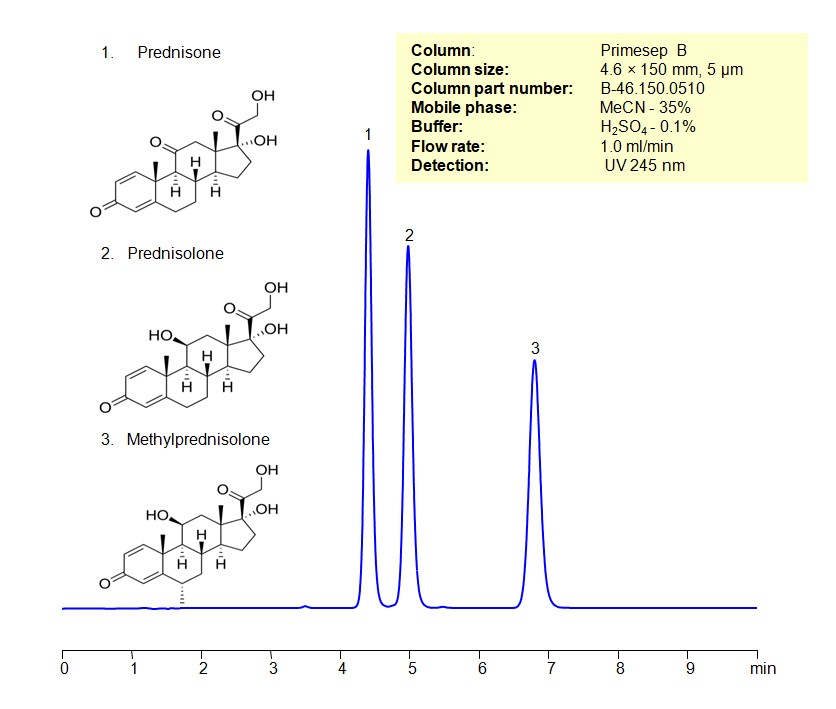
High Performance Liquid Chromatography (HPLC) Method for Analysis of Prednisone, Prednisolone, Methylprednisolone
All three compounds have potent anti-inflammatory effects, making them valuable in treating conditions where inflammation plays a role.
Prednisone:
- Mechanism of Action: Prednisone is a synthetic corticosteroid that mimics the action of cortisol, a natural steroid produced by the adrenal glands. It primarily exerts its effects by binding to glucocorticoid receptors and regulating gene expression.
- Medical Uses: It is commonly used to treat inflammatory conditions, autoimmune disorders, allergic reactions, and certain cancers.
Prednisolone:
- Derived Form: Prednisolone is the active form of prednisone. When prednisone is ingested, the liver converts it into prednisolone.
- Mechanism of Action: Similar to prednisone, it has anti-inflammatory and immunosuppressive properties.
Methylprednisolone:
- Potency: Methylprednisolone is considered to be more potent than prednisone.
- Medical Uses: It is used in various conditions, including severe allergic reactions, inflammatory conditions, and as a part of immunosuppressive regimens.
Corticosteroids can be retained, separated, and analyzed using a Primesep B mixed-mode stationary phase column. The analysis utilizes an isocratic method with a simple mobile phase consisting of water, acetonitrile (MeCN), and sulfuric acid as a buffer. Detection is achieved using UV at 245 nm
| Column | Primesep B, 4.6 x 150 mm, 5 µm, 100 A |
| Mobile Phase | MeCN/H2O – 35/35% |
| Buffer | H3PO4 -0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 245 nm |
| Class of Compounds | Corticosteroids |
| Analyzing Compounds | Prednisone, Prednisolone, Methylprednisolone |
Application Column
Primesep B
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Prednisolone
Prednisone

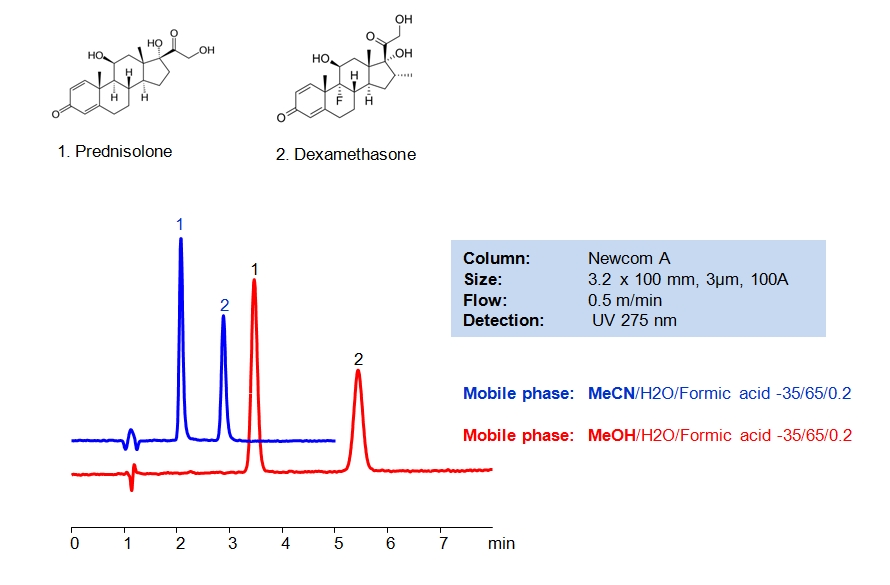
HPLC Method of Analysis for Dexamethasone and Prednisolone
June 23, 2020
Dexamethasone is a corticosteroid that is used as a replacement of the natural hormone produced by adrenal glands when the body can’t produce enough on its own. It has also been found to reduce deaths among the critically ill patients with the COVID-19. Prednisolone is another corticosteroid used to treat a variety of inflammatory conditions and has a similar structure to dexamethasone. Both steroids can be retained and separated in HPLC using Newcrom A mixed-mode column with the mobile phase consisting of either acetonitrile (ACN) or methanol (MeOH) and water with formic acid buffer. Methanol offers longer retention time compared to acetonitrile. UV detection at 275nm.
| Column | Newcrom A , 3.2×100 mm, 3 µm, 100A |
| Mobile Phase | MeCN, MeOH |
| Buffer | Formic Acid – 0.2% |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 275 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Dexamethasone, Prednisolone |
Application Column
Newcrom A
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsPrednisolone

HPLC Method for Analysis of Prednisolone
May 3, 2016
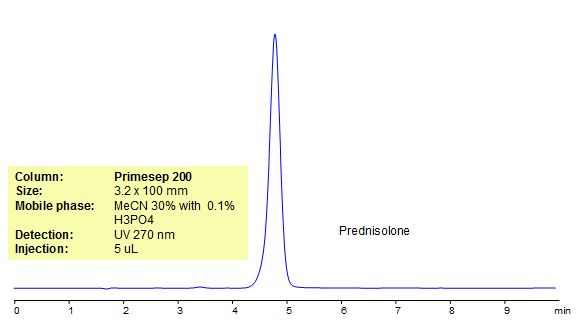
Prednisolone is a synthetic glucocorticoid with anti-inflammatory and immunomodulating properties. It can bind to and activate specific nuclear receptors, resulting in an altered gene expression and inhibition of proinflammatory cytokine production. Prednisolone is used to treat different conditions (blood problems, arthritis, immune system disorders). Primesep 200, a reverse phase column, contains embedded acidic ionizable groups and can retain Ofloxacin. The method is UV compatible and can be used as a general approach for analyzing similar compounds.
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrednisolone

USP Methods for the Analysis of Prednisolone with the Legacy L1 Column
June 21, 2012
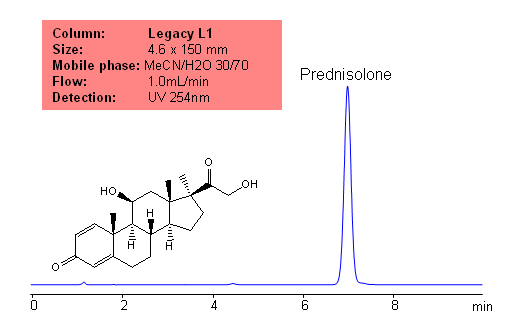
Application Notes: Prednisolone is a metabolite of prednisone. Prednisone is a common drug used to treat inflammatory diseases. The USP HPLC method for the separation of pyridoxine was developed on Legacy L1 column according to the US Pharmacopeia methodology. L1 classification is assigned to reversed-phase HPLC column containing C18 ligand. Support for the material is spherical silica gel with particles size 3-10 um and pore size of 100-120A. Resolution between critical pairs corresponds to rules and specifications of UPS.
Application Columns: Legacy L1 C18 HPLC column
Application compounds: Prednisolone
Mobile phase: MeCN/H20 30/70
Detection technique: UV
Reference: USP35: NF30
| Column | Legacy L1, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeOH/H2O – 30/70% |
| Buffer | No |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 254 nm |
| Class of Compounds |
Drug, Steroid, Hydrophobic, Ionizable |
| Analyzing Compounds | Prednisolone |
Application Column
Legacy L1
SIELC's family of Legacy columns is based on the United States Pharmacopeia's (USP) published chromatographic methods and procedures. Numerous brands have columns used in USP reference standards and methods. USP has created various designations to group together columns with similar types of packing and properties in the solid phase. SIELC's Legacy columns adhere to these strict requirements and properties, allowing you to easily replace older columns that are no longer available without needing to significantly modify your method or SOPs.
Select options
HPLC Separation of Prednisolone, Atrolactic Acid, and Ibuprofen on Mixed-Mode Column
October 4, 2010
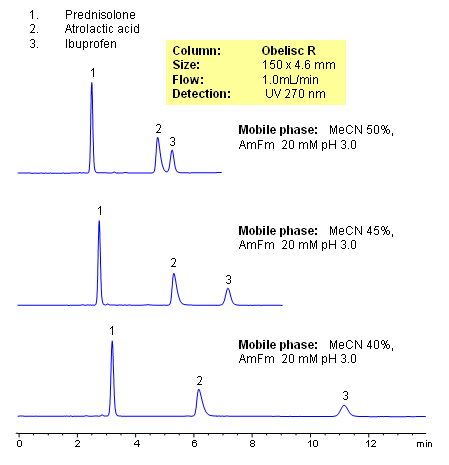
Prednisolone, atrolactic acid, and ibuprofen were separated in one HPLC run on an Obelisc R HPLC column. Obelisc R columns have C12 carbon chain and basic and acidic groups on the surface of silica gel. Compounds are separated by combination of reversed-phase, cation-exchange, and anion-exchange mechanisms. Obelisc R column can be used for retention and separation of acidic and basic hydrophilic compounds, acidic and basic hydrophobic compounds, and neutral compounds. Elution is monitored by common detection techniques like UV, ELSD, CAD and LC/MS.
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsPrednisolone

Effect of Buffer and Chemistry of Column Stationary Phase on Resolution of Neutral Compounds
April 27, 2010
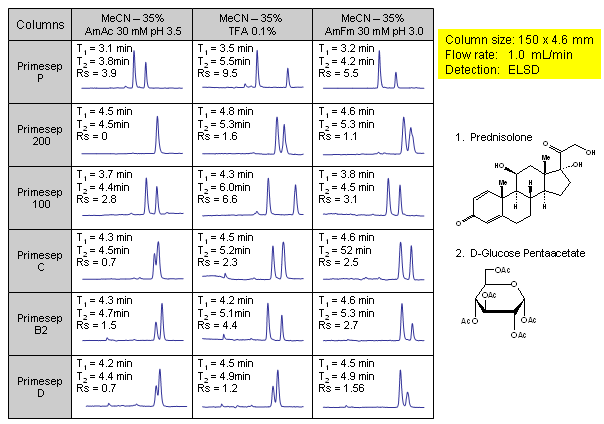
In this application, 6 various mixed-mode phases were screened for separation of two neutral compounds. Experiments were conducted on four cation-exchange mixed-mode HPLC columns (Primesep P, Primesep 200, Primesep 100, and Primesep C) and two anion-exchange mixed-mode columns (Primesep D and Primesep B2). All phases showed different selectivity towards HPLC separation of prednisolone and glucose pentaacetate. Application shows that mixed-mode chromatography is a valuable tool in separation of neutral compounds by reversed-phase mechanism. Compounds were monitored by ELSD.
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep B2
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep C
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep D
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrimesep P
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrednisolone

Effect of Buffer Concentration on Resolution of Neutral Compounds (Prednisolone and D-Glucose Pentaacetate)
April 27, 2010
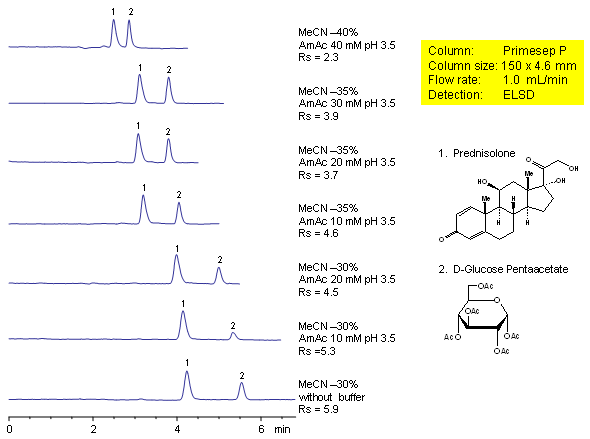
Effect of buffer concentration on retention and selectivity of HPLC separation of prednisolone and glucose pentaacetate was studied on a mixed-mode Primesep P cation-exchange column.
Application Column
Primesep P
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsPrednisolone