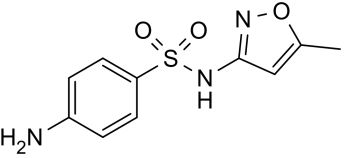
| CAS Number | 723-46-6 |
|---|---|
| Molecular Formula | C10H11N3O3S |
| Molecular Weight | 253.276 |
| InChI Key | JLKIGFTWXXRPMT-UHFFFAOYSA-N |
| LogP | 0.9 |
| Synonyms |
|
Applications:
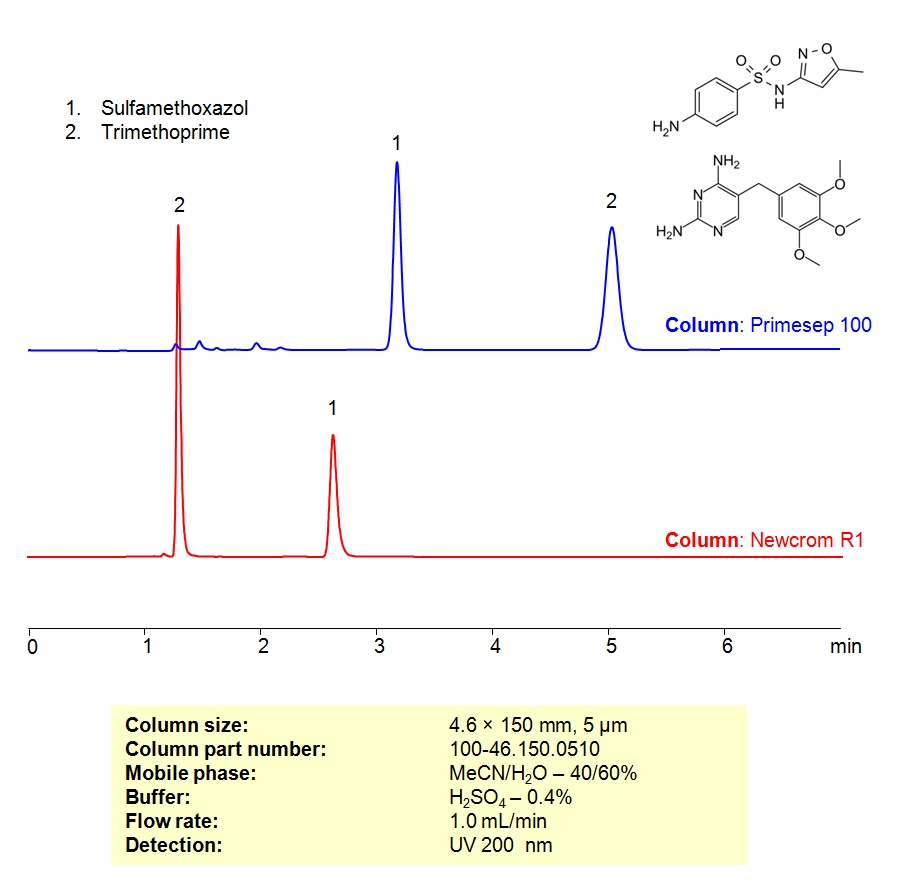
HPLC Method For Analysis Of Sulfamethoxazole and Trimethoprim on Primesep 100 Column
March 22, 2022
Separation type: Liquid Chromatography Mixed-mode
High Performance Liquid Chromatography (HPLC) Method for Analysis of Sulfamethoxazole and Trimethoprim
| Column | Primesep 100, Newcrom R1 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 40/60% |
| Buffer | H2SO4 – 0.4% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm, |
| Class of Compounds |
Drug |
| Analyzing Compounds | Sulfamethoxazole, Trimethoprim |
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsPrimesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsSulfamethoxazole
Trimethoprim

Separation of Antibiotics in Mixed-mode chromatography
May 11, 2015
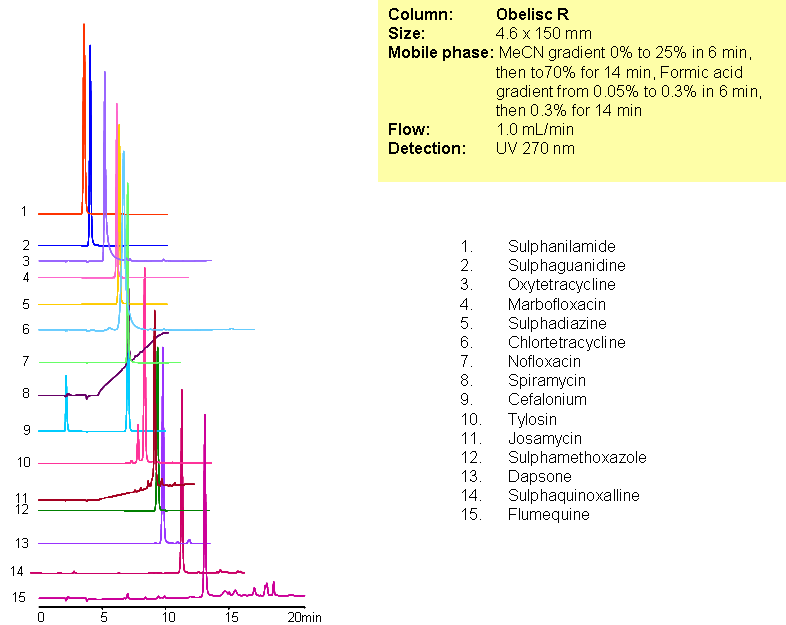
A complex mixture of sulphonamide, macrolide, tetracycline and fluoroquinolone antibiotics were separated in one run using mixed-mode chromatography with LC/MS -compatible conditions. All compounds are separated based on reversed-phase and/or ion-exchange mechanism. Method can be used for analysis of various classes of antibiotics and related impurities in different sample matrices (blood, urine, soil, waste water).
| Column | Obelisc R, 2.1×150 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 0-25%, 6 min, 25-70% 14 min |
| Buffer | Gradient Formic Acid – 0.05%-0.3%, 10 min, 14 min hold |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Antibiotic, Drug, Hydrophobic, Ionizable |
| Analyzing Compounds | Sulphanilamide, Sulphaguanidine, Oxytetracycline, Marbofloxacin, Sulphadiazine, Chlortetracycline, Nofloxacin, Spiramycin, Cefalonium, Tylosin, Josamycin, Sulphamethoxazole, Dapsone, Sulphaquinoxalline, Flumequine |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsChlortetracycline
Dapsone
Flumequine
Josamycin
Marbofloxacin
Norfloxacin
Oxytetracycline
Spiramycin
Sulfamethoxazole
Sulfonamides
Sulphadiazine
Sulphaguanidine
Sulphanilamide
Sulphaquinoxaline
Tetracycline
Tylosin
UV Detection