| CAS Number | 108-88-3 |
|---|---|
| Molecular Formula | C7H8 |
| Molecular Weight | 92.141 |
| InChI Key | YXFVVABEGXRONW-UHFFFAOYSA-N |
| LogP | 2.73 |
| Synonyms |
|
Applications:
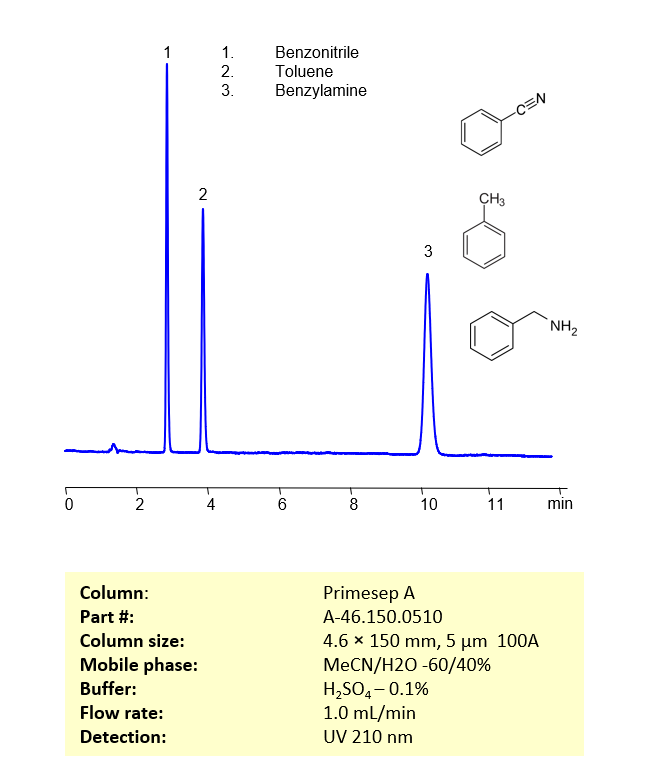
HPLC Method for Separation of Benzonitrile, Toluene and Benzylamine on Primesep A Column
May 31, 2023
HPLC Method for Analysis of Benzonitrile, Toluene, Benzylamine on Primesep A by SIELC Technologies
Separation type: Liquid Chromatography Mixed-mode
- Benzonitrile: Benzonitrile (C₆H₅CN) is an aromatic organic compound. It is a colorless liquid with an almond-like odor. Benzonitrile is used as a solvent and a chemical intermediate in the production of a variety of compounds, including pharmaceuticals, dyes, and perfumes.
- Toluene: Toluene (C₇H₈) is a colorless, water-insoluble liquid with the smell associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a CH₃ group attached to a phenyl group. It is an important organic solvent and is also used to produce benzene, and a number of consumer products including certain types of glue, paint thinners, and nail polish removers.
- Benzylamine: Benzylamine (C₆H₅CH₂NH₂) is a primary amine compound containing a benzyl group attached to the amino group. It is a colorless liquid with a faint almond-like odor. It is used as a chemical intermediate in the synthesis of a variety of substances, including pharmaceuticals, dyes, and polymers.
These chemicals are widely used in organic synthesis. Please handle these substances with care, as they can have hazardous effects. Always refer to the safety data sheets for each chemical and adhere to recommended safety procedures.
These three compounds be separate and analyzed on a reverse-phase Primesep A, 4.6 x 150 mm, 5 µm, 100 A, dual ended column with a mobile phase consisting of water, Acetonitrile (MeCN) and Sulfuric acid as a buffer modifier. This analysis method can be UV detected at 210 nm.
LOD was determined for this combination of instrument, method, and analyte, and it can vary from one laboratory to another even when the same general type of analysis is being performed.
High Performance Liquid Chromatography (HPLC) Method for Analysis of Benzonitrile, Toluene, Benzylamine
Condition
| Column | Primesep A, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O -60/40% |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV 210 nm |
| Peaks Retention Time | 2.9, 4.0, 10.2 min |
| Samples concentration (Benzonitrile, Toluene, Benzylamine) | 0.04, 0.05, 0.07 mg/ml |
| Injection volume | 3 µl |
| Sample diluent | MeCN/H2O -60/40% |
| LOD (Benzonitrile, Toluene, Benzylamine) | 27, 65, 103 ppb |
Description
| Class of Compounds | Benzene, Aromatics |
| Analyzing Compounds | Benzonitrile, Toluene, Benzylamine |
Application Column
Primesep A
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Benzylamine
Toluene

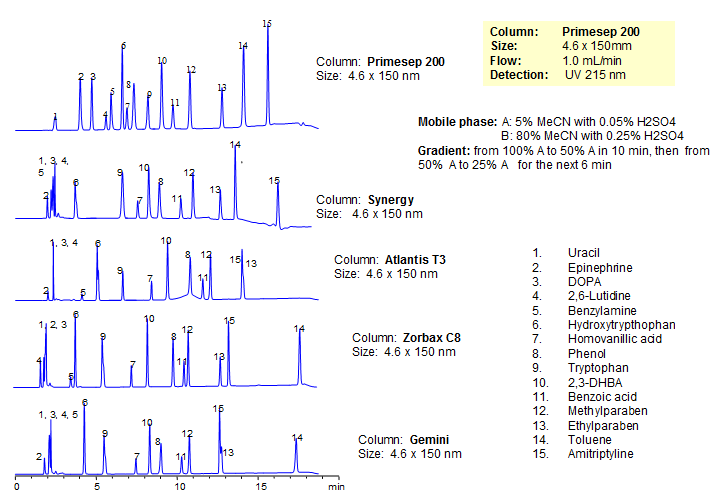
Generic Screening Method for Complex Mixtures on Primesep 200
October 15, 2015
| Column | Primesep 200, 4.6*150 mm 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 215 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Uracil, Epinephrine, DOPA, 2,6-Lutidine, Benzylamine, Hydroxytrypthophan, Homovanillic acid, Phenol, Tryptophan , 2,3-DHBA, Benzoic acid, Methylparaben, Ethylparaben, Toluene, Amitriptyline |
Application Column
Primesep 200
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
2,6-Lutidine
Amitriptyline
Benzoic Acid
Benzylamine
DOPA (3,4-dihydroxy-L-phenylalanine)
Epinephrine
Ethylparaben
Homovanillic Acid
Hydroxytryptophan
Methylparaben
Phenol
Toluene
Tryptophan
Uracil

HPLC Separation of 8 Generic Compounds on Primesep 200
March 27, 2011
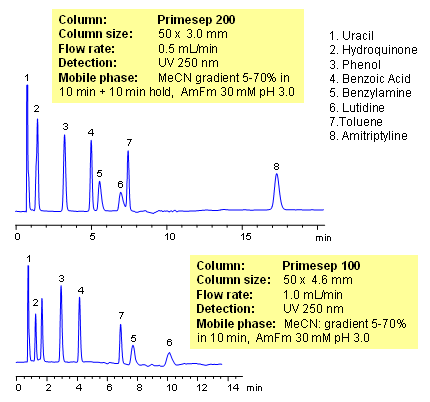
Mixed-mode HPLC columns allow to analyze compounds with drastically different properties in one run. Acidic, basic, and neutral compounds can be separated in one run using either isocratic or gradient conditions. In this application, neutral hydrophilic (uracil, phenol and hydroquinone), neutral hydrophobic (toluene), hydrophilic acidic (benzoic acid), hydrophilic basic (lutidine) and hydrophobic basic (amitriptyline) are separated using gradient of ACN. Neutral compounds are retained by reversed-phase mechanism, hydrophilic acidic compound become more hydrophobic at lower pH and retain by reversed-phase mechanism too. Basic compounds are retained by cation exchange mechanism, and hydrophobic basic compounds are retained by reversed-phase and cation-exchange mechanisms. All compounds are resolved within 17 minutes on a short column. Method can be applied to various polar and hydrophobic compounds, which can be separated on one column and in one run. Mixed-mode columns can operate in single or combination of several modes: reversed-phase, ion-exchange, ion-exclusion and HILIC. This mixed-mode HPLC column can be used as a general column for separation of wide range of compounds.
| Column | Primesep 200, Primesep 100 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm pH3.0 |
| Flow Rate | 0.5 ml/min, 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Uracil, Hydroquinone, Phenol, Benzoic Acid, Benzylamine, Lutidine, Toluene, Amitriptyline |
Application Column
Primesep 200
Column Diameter: 3.2 mm
Column Length: 50 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Benzoic Acid
Benzylamine
Hydroquinone
Lutidine
Phenol
Toluene
Uracil

HPLC Separation of 8 Generic Compounds on Primesep 100
March 27, 2011

Mixed-mode HPLC columns allow to analyze compounds with drastically different properties in one run. Acidic, basic, and neutral compounds can be separated in one run using either isocratic or gradient conditions. In this application, neutral hydrophilic (uracil, phenol and hydroquinone), neutral hydrophobic (toluene), hydrophilic acidic (benzoic acid), hydrophilic basic (lutidine) and hydrophobic basic (amitriptyline) are separated using gradient of ACN. Neutral compounds are retained by reversed-phase mechanism, hydrophilic acidic compound become more hydrophobic at lower pH and retain by reversed-phase mechanism too. Basic compounds are retained by cation exchange mechanism, and hydrophobic basic compounds are retained by reversed-phase and cation-exchange mechanisms. All compounds are resolved within 17 minutes on a short column. Method can be applied to various polar and hydrophobic compounds, which can be separated on one column and in one run. Mixed-mode columns can operate in single or combination of several modes: reversed-phase, ion-exchange, ion-exclusion and HILIC. This mixed-mode HPLC column can be used as a general column for separation of wide range of compounds.
| Column | Primesep 200, Primesep 100 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm pH3.0 |
| Flow Rate | 0.5 ml/min, 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Uracil, Hydroquinone, Phenol, Benzoic Acid, Benzylamine, Lutidine, Toluene, Amitriptyline |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 50 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Benzoic Acid
Benzylamine
Hydroquinone
Lutidine
Phenol
Toluene
Uracil

HPLC Separation of Acidic, Basic, and Neutral Compounds on Primesep 200
November 21, 2010

Primesep 100 and Primesep 200 columns can be used as a universal column for analysis of wide range of compounds. These mixed-mode reversed-phase ion-exchange HPLC columns can provide a valuable alternative to traditional reversed-phase column. Amines, amino acids, quaternary amines, and various zwitter-ions can be analyzed along with hydrophobic compounds and organic and inorganic counter-ions. In this application, 8 compounds with different hydrophobic, hydrophilic, basic and acidic properties are separated based on their properties. Primesep 100 column is a mixed-mode HPLC column with a C12 carbon chain and carboxylic acid on the surface with pKa of 1. Primesep 200 column is a mixed-mode HPLC column with a C12 carbon chain and carboxylic acid on the surface with pKa of 2. These columns can be used with 100% organic (ACN) and 100% aqueous mobile phases. This HPLC method can be adopted as a generic and robust approach for analysis of acidic, basic and neutral compounds within the same run.
| Column | Primesep 200, 3.0×50 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmFm pH3.0 |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Hormone |
| Analyzing Compounds | Uracil, Hydroquinone, Phenol, Benzoic Acid, Benzylamine, Lutidine, Toluene, Amitriptyline |
Application Column
Primesep 200
Column Diameter: 3.2 mm
Column Length: 50 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Benzoic Acid
Benzylamine
Hydroquinone
Lutidine
Phenol
Toluene
Uracil

HPLC Separation of Methyl Paraben, Benzonitrile, Propyl Paraben, and Toluene on Mixed-Mode and Reverse Phase Columns
October 4, 2010
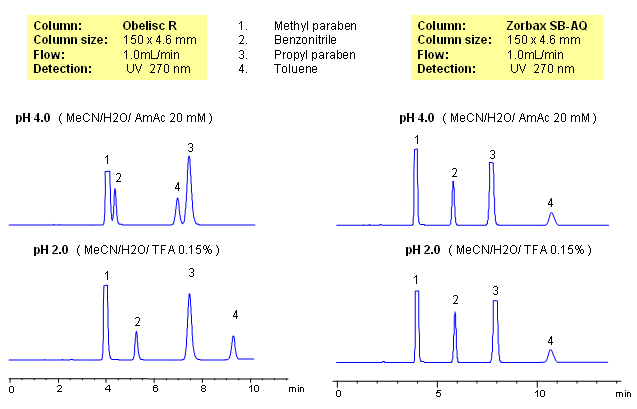
Parabens are common preservatives in pharmaceutical and cosmetic industries. They are esters of p-hydroxybenzoic acid. Method for separation of methyl paraben, propyl paraben, benzonitrile and toluene was developed on a Obelisc R column. All four compounds are neutral and are retained by reverse-phase mechanism. In case of reversed-phase stationary phase, no effect of pH is observed. Retention time for all four compounds changes on an Obelisc R column when pH is changed. pH of the mobile phase affects ionization state of stationary phase. Obelisc R column has C12 carbon chain and carboxylic acid with pKa of 4. At lower pH (pH 2, TFA), carboxylic acid of stationary phase is not ionized and thus adds hydrophobicity to stationary phase. Obelisc R column can be used for analysis of basic, acidic and neutral compounds with suitable detection techniques – UV, ELSD, CAD, LC/MS.
| Column | Obelisc R, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | AmAc, TFA |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Preservatives, Neutral |
| Analyzing Compounds | Methylparaben, Benzonitrile, Propyl paraben, Toluene |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsMethylparaben
Propylparaben
Toluene

HPLC Application for Simultaneous Separation of Amino Acids, Hydrophilic Acidic and Hydrophobic Neutral Compounds
December 6, 2007
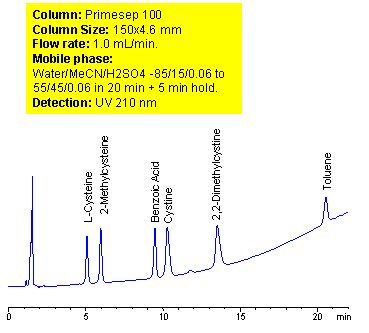
Mixed-mode chromatography allows separating, in single run, compounds with vastly different properties. A method for separation of amino acids (cysteine, methylcysteine, cystine and dimethylcysteine) in the presence of carboxylic acid (benzoic) and hydrophobic neutral compounds was developed on Primesep 100 mixed-mode column. At lower pH ionization of carboxylic acids is suppressed. Amino acids are retained as basic compound based on reverse phase and cation exchange mechanisms. Carboxylic acids are retained on this column based on weak reverse phase mechanisms. Neutral compounds are retained by reverse phase mechanism as on any other column. Retention time of basic, zwitter-ionic and hydrophobic compound can be adjusted by manipulation of mobile phase composition. ELSD, UV or LC/MS detection can be used based on the properties of analytes and mobile phase selection.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Cysteine, Methylcysteine, Cystine, Dimethylcysteine, Benzoic acid, Toluene, |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options2-Methylcysteine
Benzoic Acid
Cystine
L-Cysteine
Toluene

HPLC Separation of Amino Acids, Bases, Acids, and Neutrals on Obelisc R
March 3, 2007
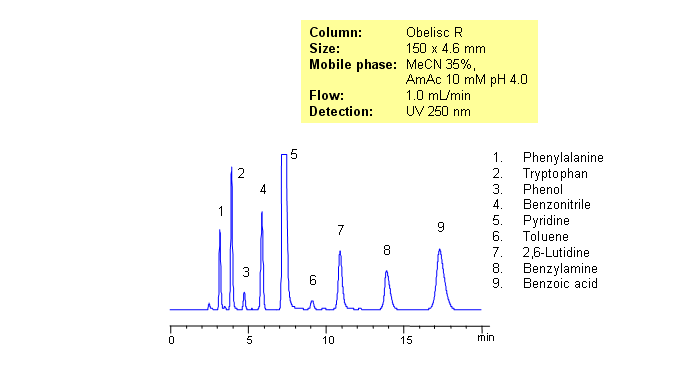
Separating basic, acidic and zwitterionic compounds in one run in reverse-phase HPLC can be very challenging. The methods might require the use of ion-pairing reagents and complex gradients that can make MS-compatibility difficult. Obelisc R column which has both positive and negative ion-pairs embedded in the stationary phase allows for fine tuning and separation of a wide range of compounds with different ionic properties. Acids, bases, amino acids and neutral compounds were separated isocratically in one run using a simple MS-compatible mobile phase of acetonitrile (ACN) and water with Ammonium Acetate (AmAc) buffer. Can also be UV detected at 250nm.
| Column | Obelisc R, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 35/65% |
| Buffer | AmAc 10 mM pH 4.0 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Acid, Bases, Neutral, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Amino acids |
Application Column
Obelisc R
SIELC has developed the Obelisc™ columns, which are mixed-mode and utilize Liquid Separation Cell technology (LiSC™). These cost-effective columns are the first of their kind to be commercially available and can replace multiple HPLC columns, including reversed-phase (RP), AQ-type reversed-phase, polar-embedded group RP columns, normal-phase, cation-exchange, anion-exchange, ion-exclusion, and HILIC (Hydrophilic Interaction Liquid Chromatography) columns. By controlling just three orthogonal method parameters - buffer concentration, buffer pH, and organic modifier concentration - users can adjust the column properties with pinpoint precision to separate complex mixtures.
Select optionsBenzoic Acid
Benzonitrile
Benzylamine
Phenol
Phenylalanine
Pyridine
Toluene
Tryptophan

Complex Mixture of Acids, Bases, Amino Acids, and Neutral Compounds
October 14, 2006
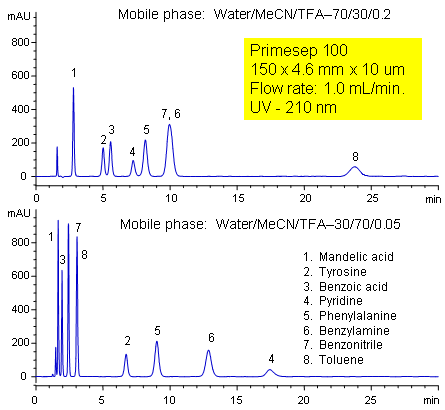
Primesep 100 separates a mixture of amino acids (tyrosine, phenylalanine), organic acids (benzoic acid, mandelic acid), amines (benzylamine, pyridine), and neutrals (benzonitrile, toluene) in one HPLC run by combining reversed-phase, cation-exchange, and polar interactions. The method is tunable and peak order can be changed significantly by adjusting acetonitrile and trifluoroacetic acid concentrations. The separation method uses a mobile phase mixture of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) and compatible with UV, mass spec (LC/MS) and evaporative light scattering (ELSD) detection.
| Column | Primesep 100, 4.6×250 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 30/70% |
| Buffer | TFA – 0.2 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Tyrosine, phenylalanine, Benzoic acid, mandelic acid, Benzylamine, Pyridine, Benzonitrile, Toluene |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsBenzoic Acid
Benzonitrile
Benzylamine
Mandelic Acid
Organic Acids
Phenylalanine
Pyridine
Toluene
Tyrosine

Hydrophobic and Hydrophilic Compound Separation
September 25, 2003
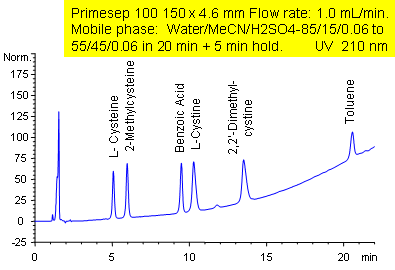
Primesep 100 separates a mixture of polar and nonpolar compounds in one analytical run. The amino acid cysteine; amino acid derivatives L-cystine, 2,2-dimethylcystine, and 2-methylcysteine; the polar acid benzoic acid; and the nonpolar neutral toluene are separated by a gradient using a combination of polar and hydrophobic interactions. The separation method uses a mobile phase mixture of water, acetonitrile (MeCN, ACN) and sulfuric acid (H2SO4) with UV detection at 210 nm.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV 210 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Nonpolar, Polar, Supplements |
| Analyzing Compounds | Cysteine, L-cystine, 2,2-dimethylcystine, 2-methylcysteine, Benzoic Acid, Toluene |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select options2-Methylcysteine
Amino Acids
Benzoic Acid
Cysteine
L-Cystine
Toluene