| CAS Number | 75-50-3 |
|---|---|
| Molecular Formula | C3H9N |
| Molecular Weight | 59.112 |
| InChI Key | GETQZCLCWQTVFV-UHFFFAOYSA-N |
| LogP | 0.16 |
| Synonyms |
|
Applications:
Mixed-Mode HPLC Separation of Tertiary Amines on Primesep 200 Column
December 6, 2007
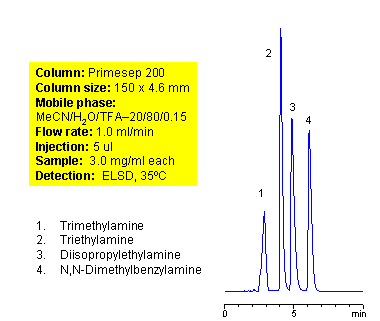
Tertiary amines are compounds derived from ammonia. Amines with short alkyl (triethylamine, trimethylamine, diisopropylethylamine) chain are not hydrophobic enough to retain well in reverse phase chromatography. Slight hydrophobicity and strong basic properties of tertiary amines provide enough interaction with mixed-mode stationary phases to guarantee good retention on a Primesep 200 column. Tertiary amines can be separated by dual interaction based on minimal difference in hydrophobic and basic properties. This application can be used for analysis of complex mixtures of tertiary amines. ELSD and LC/MS detection can be applied. Various buffers compatible with specified detection technique can be employed (TFA for ELSD, ammonium acetate or ammonium formate for LC/MS) Compounds with the nitrogen atom next to carbonyl are called amides and are not basic in nature. Mixed-mode cation-exchange stationary phases are not interacting with amides by ion-exchange mechanism.
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsN,N-Dimethylbenzylamine
Triethylamine
Trimethylamine

Separation of Simple Tertiary Amines
September 25, 2005
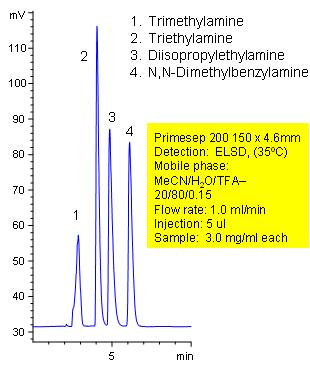
Primesep 200 separates simple tertiary amines (trimethylamine, triethylamine, diisopropylethylamine, N,N-dimethylbenzylamine) by cation exchange and reversed phase. Primesep 200 has an embedded anionic functional group which helps retain cations by polar and ion-exchange mechanisms. Excellent peak shape results with a mass spec compatible mobile phase of water, acetonitrile (MeCN, ACN) and trifluoracetic acid (TFA) with evaporative light scattering detection (ELSD).
Application Column
Primesep 200
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsN,N-Dimethylbenzylamine
Tertiary Amines
Triethylamine
Trimethylamine