| CAS Number | 69-89-6 |
|---|---|
| Molecular Formula | C5H4N4O2 |
| Molecular Weight | 152.114 |
| InChI Key | LRFVTYWOQMYALW-UHFFFAOYSA-N |
| LogP | -0.730 |
| Synonyms |
|
Applications:
HPLC Method for Analysis mixture of Xanthinesand Uric Acid BIST B+ by SIELC Technologies
November 16, 2022
HPLC Method for Analysis mixture of Xanthines and Uric Acid BIST B+ by SIELC Technologies.
Separation type: Hydrophilic interaction liquid chromatography (HILIC)
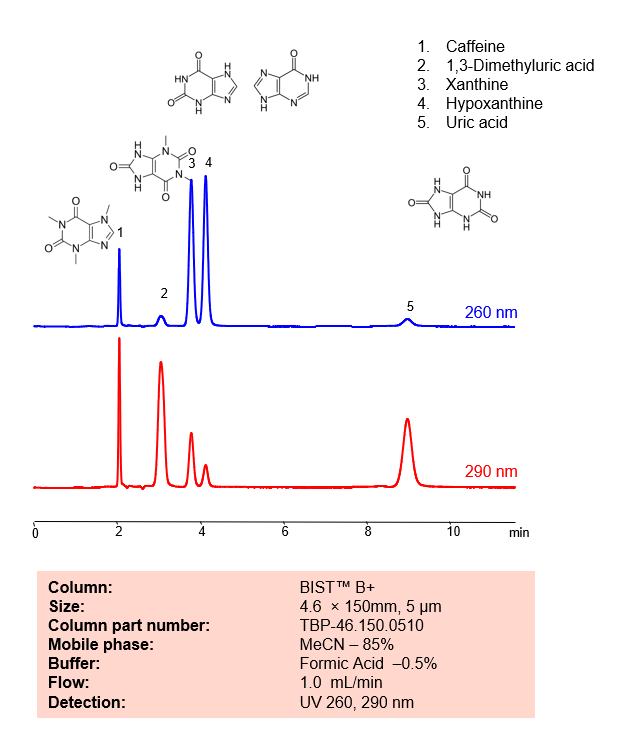
Xanthines and uric acid are related compounds in the body and both are involved in the metabolism of purines.
Xanthines are a group of alkaloids that are widely distributed in plants, and also occur in the tissues and fluids of animals. They are known to stimulate the central nervous system and cardiac muscle, and also have diuretic effects. Some commonly known xanthines include caffeine (found in coffee, tea, and chocolate), theobromine (found in cocoa and chocolate), and theophylline (used as a drug in the treatment of respiratory diseases like asthma).
In the body, xanthines are intermediates in the degradation of adenosine monophosphate to uric acid. This metabolic pathway starts with adenosine monophosphate (AMP), which is deaminated to form inosine monophosphate (IMP). IMP is then converted into a xanthine known as hypoxanthine. Hypoxanthine is then oxidized to xanthine, and finally, xanthine is further oxidized to uric acid. Both of the oxidation steps are catalyzed by the enzyme xanthine oxidase.
Uric acid and Xanthines can be retained, analyzed, and separated using an isocratic analytical method on a BIST B+ column. The simple mobile phase for this method comprises water, acetonitrile (MeCN), and formic acid as an ionic modifier. The analytical method can be monitored with UV detection at 260 nm, an Evaporative Light Scattering Detector (ELSD), or any other evaporative detection method such as Charged Aerosol Detection (CAD) or Electrospray Ionization Mass Spectrometry (ESI-MS)
Condition
| Column | BIST B+, 4.6 x 150 mm, 10 µm, 100 A |
| Mobile Phase | MeCN – 85% |
| Buffer | FA – 0.5% |
| Flow Rate | 1.0 ml/min |
| Detection | UV 260, 290 nm |
| Peak Retention Time | 2.01, 3.02, 4.2, 9.09 min |
Description
| Class of Compounds | Acid, Xanthines |
| Analyzing Compounds | Uric Acid, Caffeine, 1,3-Dimethyluric acid, Xanthine, Hypoxanthine |
Application Column
BIST B+
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 10 µm
Pore Size: 100 A
Caffeine
Hypoxanthine
Uric acid
Xanthine

HPLC Separation of Caffeine, 3- Methylxanthine, 1- Methylxanthine, Xanthine
June 15, 2012
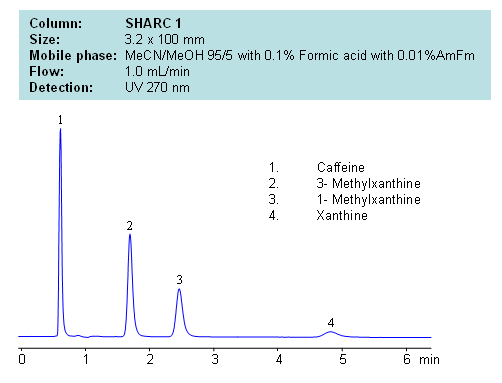
Application Notes: Xanthines are polar neutral compounds which are hard to retain and separate by traditional reversed-phase chromatography. However a hydrogen bonding method makes separation possible due to an observable correlation between the number of hydrogens available for interaction and retention time. Molecules with no hydrogens available for interactions retain less, and compound with multiple hydrogen donors retain the most. Retention time can be controlled by changing ratio of ACN:MeOH. Other protic and aprotic solvents can be used to control retention time and selectivity of separation.
Application Columns: SHARC 1, 3.2×100 mm, 5 um, 100A, To learn more about SHARC 1 columns click here. To order this column click here. To see more chromatographic separations check our web site.
Application Compounds: Caffeine, 3-methylxanthine, 1-methylxanthine, and xanthine
| Column | Sharc 1, 3.2×100 mm, 5 µm, 100A |
| Mobile Phase | MeCN/MeOH |
| Buffer | AmFm, Formic acid |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements |
| Analyzing Compounds | Caffeine, 3- Methylxanthine, 1- Methylxanthine, Xanthine |
Application Column
SHARC 1
The SHARC™ family of innovative columns represents the first commercially available columns primarily utilizing separation based on hydrogen bonding. SHARC stands for Specific Hydrogen-bond Adsorption Resolution Column. Hydrogen bonding involves an interaction or attraction between a bound hydrogen atom and molecules containing electronegative atoms, such as oxygen, nitrogen, and fluorine.
Select options3-Methylxanthine
Caffeine
Xanthine

HILIC Retention of Polar Compounds on Primesep S
August 22, 2010
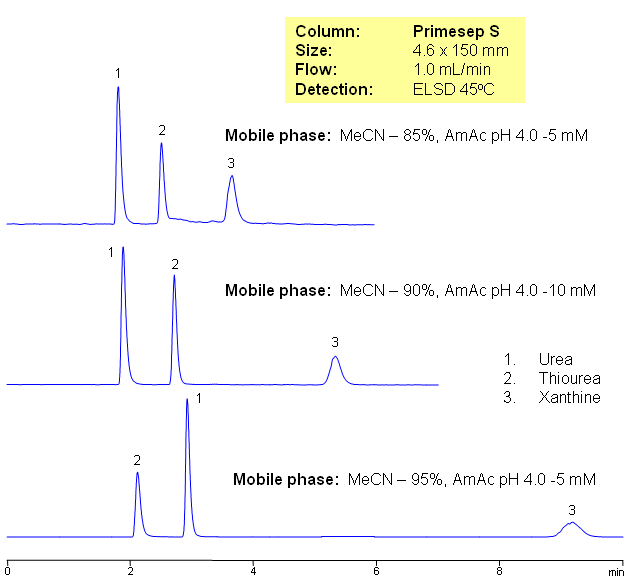
Three neutral polar compounds (urea, thiourea, and xanthine) are separated on a Primesep S HILIC cation-exchange column with LC/MS compatible mobile phase. The retention time is controlled by the amount of acetonitrile in the mobile phase.
Application Column
Primesep S
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsUrea
Xanthine