| CAS Number | 338-69-2 |
|---|---|
| Molecular Formula | C3H7NO2 |
| Molecular Weight | 89.094 |
| InChI Key | QNAYBMKLOCPYGJ-UWTATZPHSA-N |
| LogP | -1.86 |
| Synonyms |
|
Applications:
New HPLC Amino Acids Separation Compatible With Carbon Dating Technique
February 11, 2020
Hydroxyproline seems to be the most promising amino acid used in carbon dating when isolated from bone collagen. Separation of amino acids is challenging, especially without the use of ions or inorganic buffers that can interfere with Mass spectrometer (MS) or contaminate the sample with modern carbon. Amino acids are also not retained in reverse-phase chromatography. The ideal solution would be using water only to separate the amino acids. This would allow a direct coupling to MS. We were able to separate hydroxyproline from proline and other simple amino acids like glycine and alanine in HPLC on Newcrom AH column using water only as a mobile phase. Using water also allowed UV detection at 205 nm which can’t be done if using a buffer based on acetic or formic acid.
See more information on radiocarbon dating here.
The same method can be modified to get symmetrical peaks and higher efficiency if a mobile phase with ionic modifier such as formic acid is used.
| Column | Newcrom AH, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 205 nm, CAD |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | Alanine, Glycine, Proline, Hydroxyproline |
Application Column
Newcrom AH
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsD-Alanine
Glycine
Hydroxyproline
Proline

HPLC Separation of a Mixture of Non-Essential Amino Acids, such as L-Aspartic Acid, L-Serine, L-Glutamic Acid, and L-Alanine on Primesep 100 Column
March 11, 2019
High Performance Liquid Chromatography (HPLC) Method for Analysis of Non-Essential Amino Acids on Primesep 100 by SIELC Technologies
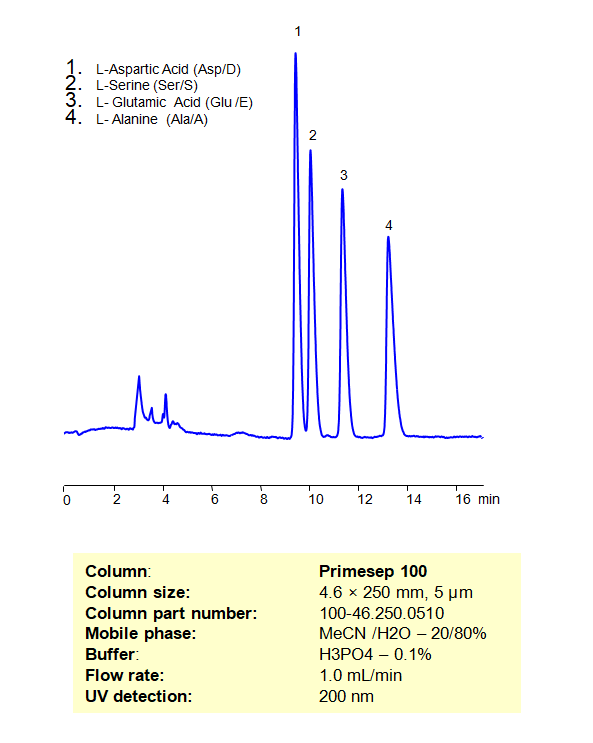
High Performance Liquid Chromatography (HPLC) Method for Analyses of Non-Essential Amino Acids
| Column | Primesep 100, 4.6 x 250 mm, 5 µm, 100 A |
| Mobile Phase | MeCN/H2O – 20/80% |
| Buffer | H3PO4 – 0.1% |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 200 nm |
| Class of Compounds | Drug, Acid, Hydrophilic, Ionizable, Vitamin, Supplements, Amino acid |
| Analyzing Compounds | L-Aspartic Acid (Asp/D), L-Serine (Ser/S), L-Glutamic Acid Glu/E), L-Alanine Ala/A) |
Amino acids are the building blocks of proteins. Based on their dietary requirement, they are classified into essential and non-essential amino acids. Essential amino acids cannot be synthesized by the human body in sufficient quantities and must be obtained from the diet. Non-essential amino acids, on the other hand, can be synthesized by the body and are not dependent on dietary intake.
It’s worth noting that while these amino acids are considered “non-essential” for adults under normal circumstances because the body can synthesize them, there are situations where some may become “conditionally essential.” This means that under certain conditions like illness, stress, or trauma, the body might not produce them in sufficient quantities, and dietary intake becomes necessary. Arginine, for instance, is considered conditionally essential, especially during periods of rapid growth, illness, or trauma.
Amino acids can be retained, separeted and analyzed on a Primesep 100 mixed-mode stationary phase column using an isocratic analytical method with a simple mobile phase of water, Acetonitrile (MeCN), and a phosphoric acid (H3PO4) as a buffer. This analysis method can be detected in the UV regime at 200 nm.
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 250 mm
Particle Size: 5 µm
Pore Size: 100 A
Amino Acids
Aspartic Acid
D-Alanine
DL-Alanine
GLU (L-Glutamic acid)
Glutamic Acid
Serine

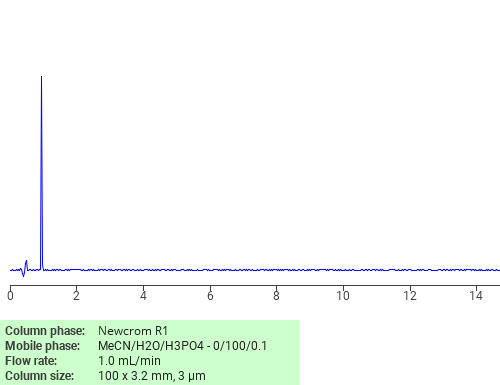
Separation of D-Alanine on Newcrom R1 HPLC column
February 16, 2018
D-Alanine can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains an acetonitrile (MeCN), water, and phosphoric acid. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particles columns available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation impurities in preparative separation. It also suitable for pharmacokinetics.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select options