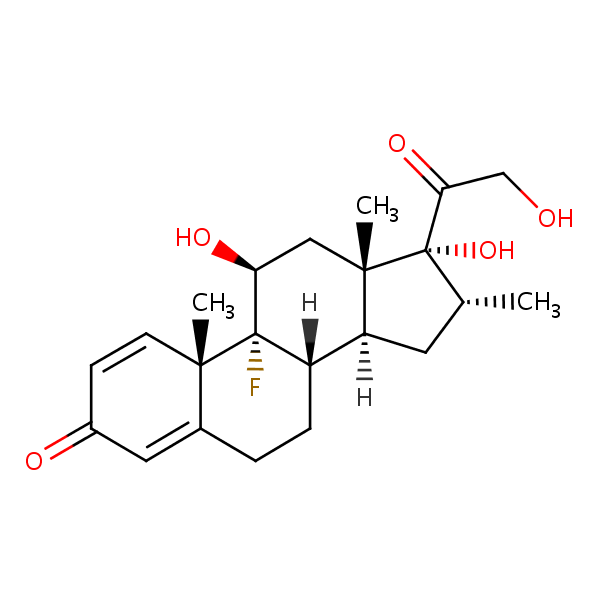
| CAS Number | 50-02-2 |
|---|---|
| Molecular Formula | C22H29FO5 |
| Molecular Weight | 392.467 |
| InChI Key | UREBDLICKHMUKA-CXSFZGCWSA-N |
| LogP | 1.83 |
| Synonyms |
|
Applications:
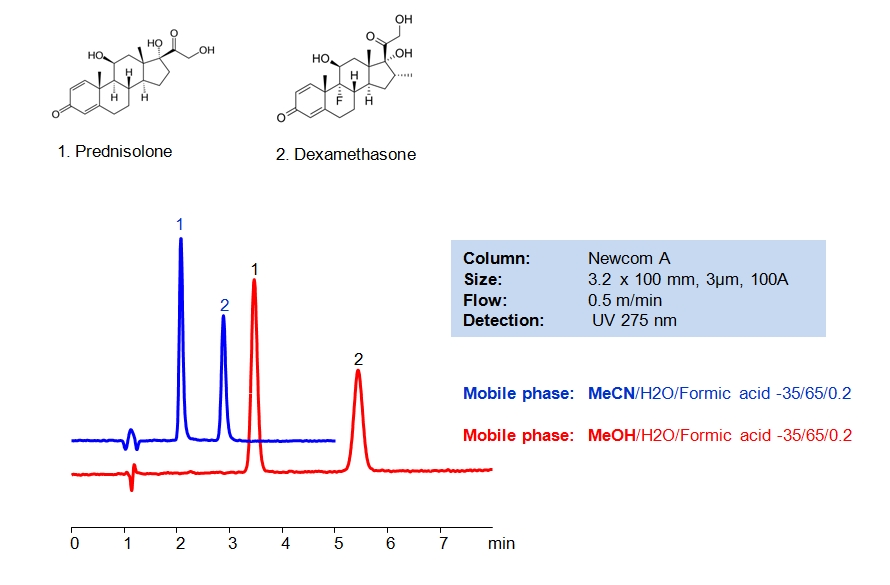
HPLC Method of Analysis for Dexamethasone and Prednisolone
June 23, 2020
Dexamethasone is a corticosteroid that is used as a replacement of the natural hormone produced by adrenal glands when the body can’t produce enough on its own. It has also been found to reduce deaths among the critically ill patients with the COVID-19. Prednisolone is another corticosteroid used to treat a variety of inflammatory conditions and has a similar structure to dexamethasone. Both steroids can be retained and separated in HPLC using Newcrom A mixed-mode column with the mobile phase consisting of either acetonitrile (ACN) or methanol (MeOH) and water with formic acid buffer. Methanol offers longer retention time compared to acetonitrile. UV detection at 275nm.
| Column | Newcrom A , 3.2×100 mm, 3 µm, 100A |
| Mobile Phase | MeCN, MeOH |
| Buffer | Formic Acid – 0.2% |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 275 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Dexamethasone, Prednisolone |
Application Column
Newcrom A
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select optionsPrednisolone

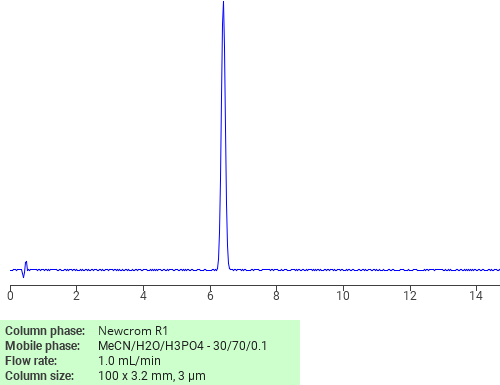
Separation of Dexamethasone on Newcrom R1 HPLC column
February 16, 2018
Dexamethasone is a corticosteroid used to treat rheumatic problems, skin diseases, and allergies, and others. It works by stimulating the glucocorticoid receptor. Dexamethasone can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains acetonitrile (MeCN), water, and phosphoric acid. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particle columns are available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation of impurities in preparative separation. It also suitable for pharmacokinetics.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
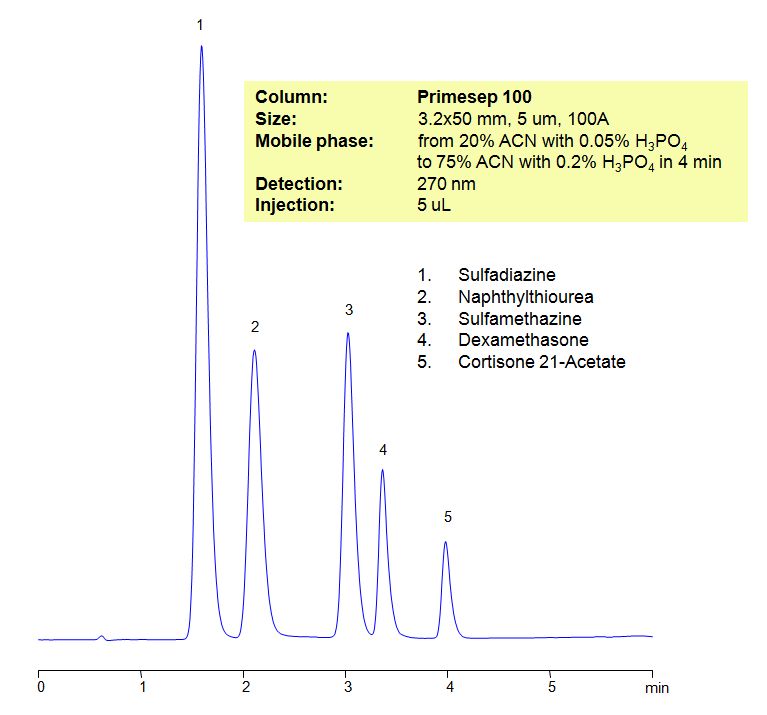
Select optionsHPLC Method for Analysis of Sulfadiazine, Naphthylthiourea, Sulfamethazine, Dexamethasone, Cortisone 21-Acetate
July 11, 2017
Sulfadiazine is an antibiotic used to prevent rheumatic fever, chancroid, chlamydia and infections by Haemophilus influenzae. There are side effects to the use of the drug which include, but not limited to: nausea, headache, rash, depression.
| Column | Primesep 100, 3.2×50 mm, 5 µm, 100A |
| Mobile Phase | Gradient MeCN – 20-75%, 4 min |
| Buffer | Gradient H3PO4 – 0.05-0.2%, 4 min |
| Flow Rate | 0.5 ml/min |
| Detection | UV, 270 nm |
| Class of Compounds |
Drug, Acid, Hydrophilic, Ionizable |
| Analyzing Compounds | Sulfadiazine, Naphthylthiourea, Sulfamethazine, Dexamethasone, Cortisone 21-Acetate |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsDexamethasone
Naphthylthiourea
Sulfadiazine
Sulfamethazine