| CAS Number | 564-25-0 |
|---|---|
| Molecular Formula | C22H24N2O8 |
| Molecular Weight | 444.441 |
| InChI Key | JBIWCJUYHHGXTC-AKNGSSGZSA-N |
| LogP | -0.0200 |
| Synonyms |
|
Applications:
UV-Vis Spectrum of Doxycycline
July 11, 2024
Access the UV-Vis Spectrum SIELC Library
If you are looking for optimized HPLC method to analyze Doxycycline check our HPLC Applications library
For optimal results in HPLC analysis, it is recommended to measure absorbance at a wavelength that matches the absorption maximum of the compound(s) being analyzed. The UV spectrum shown can assist in selecting an appropriate wavelength for your analysis. Please note that certain mobile phases and buffers may block wavelengths below 230 nm, rendering absorbance measurement at these wavelengths ineffective. If detection below 230 nm is required, it is recommended to use acetonitrile and water as low UV-transparent mobile phases, with phosphoric acid and its salts, sulfuric acid, and TFA as buffers.

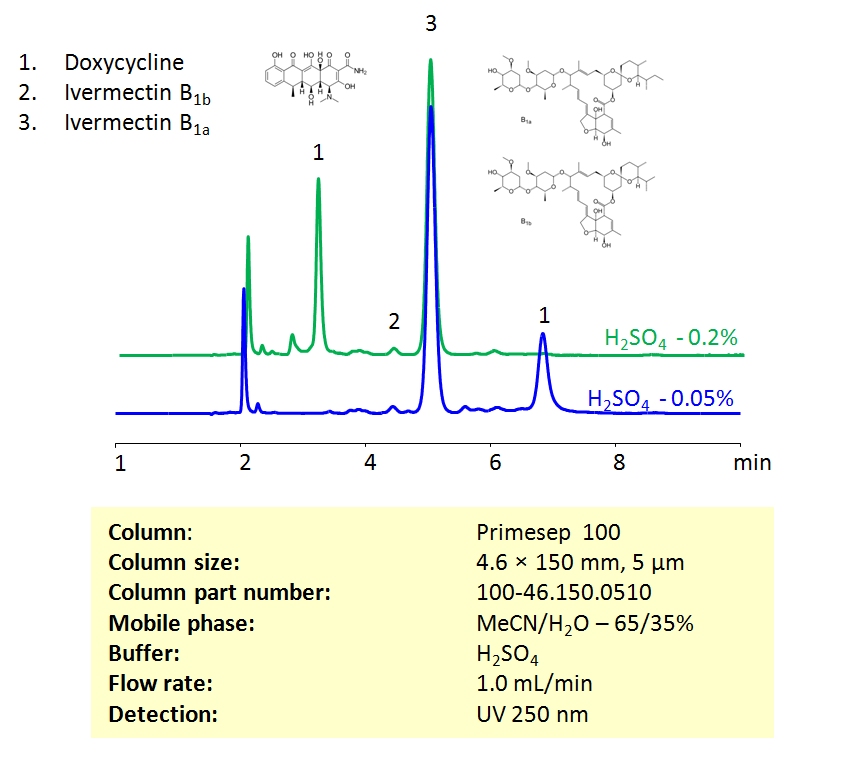
HPLC Method For Analysis Of Ivermectin and Doxycycline on Primesep 100 Column
August 31, 2021
HPLC Method for Ivermectin B1a, Doxycycline, Ivermectin B1b on Primesep 100 by SIELC Technologies
High Performance Liquid Chromatography (HPLC) Method for Analysis of Ivermectin and Doxycycline
Ivermectin is an anti-parasitic drug. 1A variation has the chemical formula C48H74O14. 1b has the chemical formula C47H72O14. It is often used to treat or prevent parasites in animals and humans. In dogs, it is routinely used to treat heartworm, though, certain breeds, can be severely poisoned by this medication. You can find detailed UV spectra of Ivermectin B1a and information about its various lambda maxima by visiting the following link.
Doxycycline is a broad spectrum antibiotic with the chemical formula C22H24N2O8. Outside of being an antibiotic, it is also said o have anti-inflammatory and anti-angiogenic properties. It is used primarily to treat Lyme disease. chronic prostatitis, sinusitis, pelvic inflammatory disease, acne, rosacea, and rickettsia. You can find detailed UV spectra of Doxycycline and information about its various lambda maxima by visiting the following link.
Ivermectin and Doxycycline can be retained and separated on the Primesep 100 mixed-mode column using an isocratic analytical method with a simple mobile phase of water, acetonitrile (MeCN, ACN), and sulphuric acid (H2SO4) buffer. The analysis method can be UV detected at 250 nm.
| Column | Primesep 100, 4.6 x 150 mm, 5 µm, 100 A, dual ended |
| Mobile Phase | MeCN/H2O – 65/35% |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Antibiotics |
| Analyzing Compounds | Ivermectin B1a, Doxycycline, Ivermectin B1b |
Application Column
Primesep 100
Column Diameter: 4.6 mm
Column Length: 150 mm
Particle Size: 5 µm
Pore Size: 100 A
Column options: dual ended
Ivermectin B1a
Ivermectin B1b

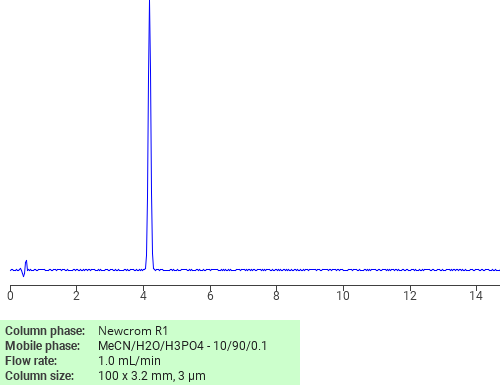
Separation of Doxycycline on Newcrom R1 HPLC column
February 16, 2018
Doxycycline can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains an acetonitrile (MeCN), water, and phosphoric acid. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particles columns available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation impurities in preparative separation. It also suitable for pharmacokinetics.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select options