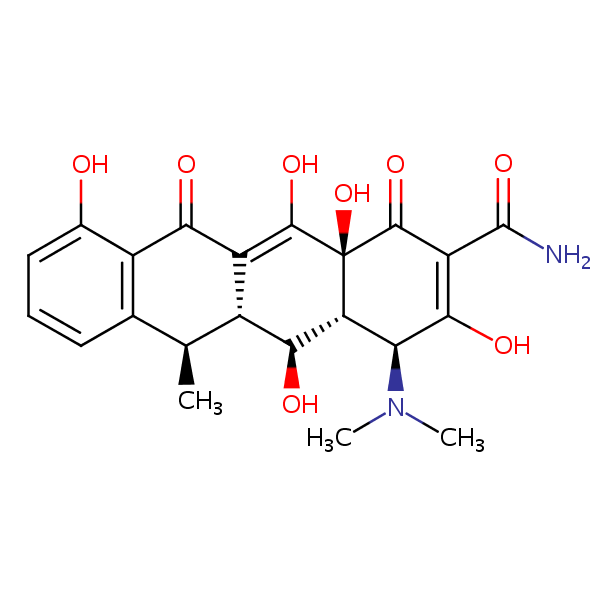
| CAS Number | 564-25-0 |
|---|---|
| Molecular Formula | C22H24N2O8 |
| Molecular Weight | 444.441 |
| InChI Key | JBIWCJUYHHGXTC-AKNGSSGZSA-N |
| LogP | -0.0200 |
| Synonyms |
|
Applications:
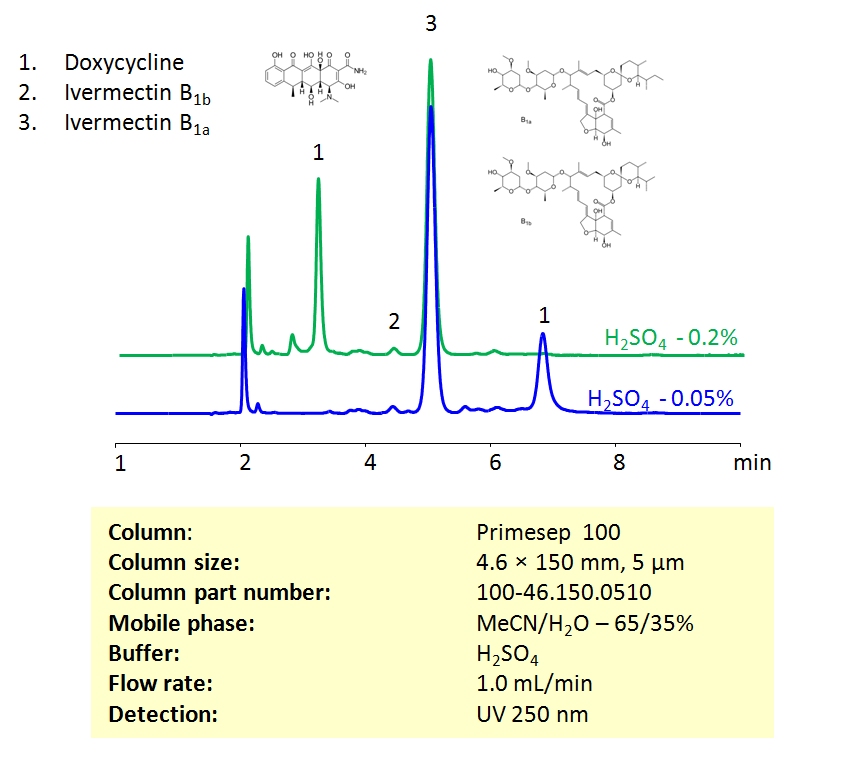
HPLC Method For Analysis Of Ivermectin and Doxycycline on Primesep 100 Column
August 31, 2021
Separation type: Liquid Chromatography Mixed-mode
High Performance Liquid Chromatography (HPLC) Method for Analysis of Ivermectin and Doxycycline
vIvermectin is an anti-parasitic drug that is often used to treat or prevent parasites in animals and humans. Ivermectin and Doxycycline can be retained and separated on the Primesep 100 mixed-mode column using an isocratic analytical method with a simple mobile phase of water, acetonitrile (MeCN, ACN), and sulphuric acid (H2SO4) buffer. The analysis method can be UV detected at 250 nm.
Ivermectin and Doxycycline can be retained and separated on the Primesep 100 mixed-mode column using an isocratic analytical method with a simple mobile phase of water, acetonitrile (MeCN, ACN), and sulphuric acid (H2SO4) buffer. The analysis method can be UV detected at 250 nm.
| Column | Primesep 100, 4.6×150 mm, 5 µm, 100A |
| Mobile Phase | MeCN/H2O – 65/35% |
| Buffer | H2SO4 |
| Flow Rate | 1.0 ml/min |
| Detection | UV, 250 nm |
| Class of Compounds |
Drug, Antibiotics |
| Analyzing Compounds | Ivermectin, Doxycycline |
Application Column
Primesep 100
The Primesep family of mixed-mode columns offers a wide variety of stationary phases, boasting unprecedented selectivity in the separation of a broad array of chemical compounds across multiple applications. Corresponding Primesep guard columns, available with all stationary phases, do not require holders. SIELC provides a method development service available to all customers. Inquire about our specially-tailored custom LC-phases for specific separations.
Select optionsIvermectin B1a
Ivermectin B1b

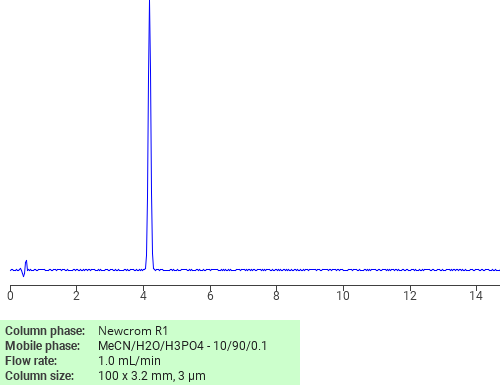
Separation of Doxycycline on Newcrom R1 HPLC column
February 16, 2018
Doxycycline can be analyzed by this reverse phase (RP) HPLC method with simple conditions. The mobile phase contains an acetonitrile (MeCN), water, and phosphoric acid. For Mass-Spec (MS) compatible applications the phosphoric acid needs to be replaced with formic acid. Smaller 3 µm particles columns available for fast UPLC applications. This liquid chromatography method is scalable and can be used for isolation impurities in preparative separation. It also suitable for pharmacokinetics.
Application Column
Newcrom R1
The Newcrom columns are a family of reverse-phase-based columns. Newcrom A, AH, B, and BH are all mixed-mode columns with either positive or negative ion-pairing groups attached to either short (25 Å) or long (100 Å) ligand chains. Newcrom R1 is a special reverse-phase column with low silanol activity.
Select options